Things to consider
- Before bleaching, be sure to pay attention to the material of the product and the symbols on the label. It should indicate at what temperature the laundry can be washed. This is important because many of the methods below use boiling water. For example, synthetics cannot be cleaned in hot water, unlike linen and cotton fabrics.
- If the item is expensive and you are afraid to experiment, it is better to take it to the dry cleaner.
- If the chosen method did not help the first time, then after washing you can repeat the bleaching using the same or a different method.
Advantages and disadvantages of bleaching laundry with hydrogen peroxide
Benefits of bleaching white fabrics with hydrogen peroxide:
- low cost;
- removal of old plaque and yellowness;
- ease of use;
- preservation of the structure of the material;
- application for delicate fabrics;
- returning washed laundry to its original appearance;
- removing stains from deodorant, sweat and oil;
- availability of the drug.
The only downside to bleaching with hydrogen peroxide is the smell. Replace 1 tbsp. l. 3% peroxide can be 1 tablet of hydroperite. The effects of the drugs are the same.
Attention! The whitening effect is achieved by directly applying the product to the stain. The matter is saturated with oxygen, which breaks down the dirt.
Peroxide is a readily available antiseptic. The drug is sold in any pharmacy in the country. For bleaching, use a 3% peroxide solution. Usual composition concentration: 2 tbsp. l. drug per 10 liters of hot water.
We recommend reading: How to remove brilliant green from linoleum
How to bleach white clothes with commercial bleach
- What does it cope with? With almost everything: eliminates stains, yellowness, grayness and even helps restore color to faded items.
- What fabrics is it suitable for? Depends on the composition.
There are a large number of different bleaches, chlorine- and oxygen-containing, on store shelves.
Products with chlorine work, but can thin the fabric and ruin the color. They are used only for linen and cotton.
Therefore, it is better to give preference to oxygen bleaches. They are suitable for all fabrics, even delicate ones, and do not require high temperatures. This is important if the item cannot be washed in hot water.
How to use bleaches may vary, so follow the instructions on the package. Such products and brands as “Persol”, ACE, “BOS”, “Ushasty Nyan”, Vanish, Amway have proven themselves to be excellent.
Most often, things need to be soaked for several hours in water with bleach and washed. Or add the product directly to the washing machine along with the powder.
If the stains are strong and the fabric allows it, you can boil the laundry with bleach for at least 15 minutes and wash it in a machine.
Possibility of combination with other means
In addition to soda and hydrogen peroxide, other means are used to whiten things, which can be safely combined with the first two:
- table salt (suitable for synthetic fabrics, wool and silk);
- ammonia (cotton, linen, wool, silk, delicate fabrics);
- potassium permanganate (suitable for all types of fabric);
- mustard (suitable for all types of fabric).
You cannot add any professional bleaches (alkaline, chlorine, oxygen) to soda or hydrogen peroxide in order to enhance the effect of the former. This can lead to unforeseen consequences, such as yellowing or deterioration of the fabric.
How to bleach white clothes with a mixture of powder, bleach, oil and soda
- What does it cope with? This is a very effective way to combat yellowness and all kinds of stains. The product can remove even very old stains.
- What fabrics is it suitable for? For cotton and linen.
Pour 1 cup (250 ml volume) of laundry detergent, 4 tablespoons of good powder bleach and 1 tablespoon of baking soda into a saucepan. Pour in 4 tablespoons of sunflower oil.
Pour 10 liters of boiling water, stir and put dry things there. The quantity of necessary components can be changed, maintaining the proportions.
Place the pan on low heat for 15–20 minutes and stir the laundry occasionally. Remove from heat and let sit overnight or 6-8 hours. Then wash the items in the machine.
Method number 6: “Whiteness”
If your white things have acquired a yellowish tint, then they are giving you a sign that it is time to use “Whiteness”. This product is suitable for lightening dense fabrics, but for thin tulle or delicate linen this bleach is dangerous. Let's learn to use Whiteness.
- Heat the water to a temperature of + 35 C.
- Pour 15 ml of “Belizna” into heated water.
- Add 20 g of washing powder.
- Immerse the laundry in the prepared solution and let it sit for 15 minutes.
That’s it, the laundry can be washed in a stylish machine in the “Fast 30” or “Rinse” mode. And another good folk remedy for whitening things involves boiling: add 15-20 ml of “Whiteness” and 20 g of washing powder to 4 liters of clean water, boil the laundry for 25 minutes. After boiling, the laundry is rinsed in plenty of clean water.
How to bleach white things with laundry soap and soda
- What does it cope with? This method is as effective as the previous one. It helps perfectly even with old stains and yellowness.
- What fabrics is it suitable for? For cotton and linen.
Place the laundry in a container with three liters of warm water. Rub each item thoroughly with laundry soap. Add 3 tablespoons of baking soda, stir and leave for several hours.
Place the pan over low heat and simmer for about 1 hour, stirring occasionally. The liquid will become colored as it absorbs contaminants.
Rinse the laundry in warm water. Do this until it comes out clean.
General information
About peroxide
Only a few of the possible ways to use peroxide were listed. However, peroxide is most often used in the household, for example, it effectively whitens laundry. This method is very affordable and economical for returning things to their former freshness and snow-whiteness.
Hydrogen peroxide. This drug has many useful qualities.
It is often used in medicine as a means to disinfect wounds, although it can also be used in other cases, such as:
— treatment of diseases of the ears and oral cavity;
— getting rid of mold and fungal infections;
- getting rid of skin blemishes;
- hair lightening, etc.
Pros and cons of this type of whitening
Hydrogen peroxide is universal; it is suitable for bleaching all fabrics. You can purchase this product at any pharmacy. Textile fibers are not damaged by such fabric bleaching. This method can also be used to bleach delicate items made of wool or silk. To bleach fabrics, you can only use a 3% peroxide solution, which is made by mixing 2 tbsp. l. ammonia and peroxide solution and 10 liters of water. Items that need to be bleached are soaked in the prepared solution for half an hour and then washed as usual.
REFERENCE! You can return things to their original appearance, which turns gray and fades with constant wear and frequent washing, using this method. It gets rid of traces of sweat, vegetable oil, and antiperspirant.
Pros of bleaching things with peroxide:
The whitening effect is achieved through the direct action of peroxide on stains, while it saturates the material with oxygen and breaks down dirt.
An analogue of peroxide is hydroperite in tablets. Instead of one spoon of peroxide, you will need one tablet of hydroperite. The action of these drugs is the same, but hydroperite differs in the presence of urea in the composition.
How to bleach white clothes with boric acid
- What does it cope with? A solution of boric acid is not able to remove yellowness. But it will rid things of grayness and return them to their former whiteness. For example, it will refresh old washed bed linen.
- What fabrics is it suitable for? For cotton and linen, as well as for synthetics, if it is not boiled.
Dissolve 2 tablespoons of boric acid in two liters of warm water. Leave the items in the mixture for 2 hours, stirring occasionally, and wash in the machine.
If the stains are old, boiling will help. To do this, place the container with things and the prepared solution on low heat. There is no need to soak the laundry. Boil for about 1 hour and throw in the washing machine.
Features of bleaching laundry with hydrogen peroxide
White clothes look noble. But maintaining her ideal appearance for as long as possible is quite difficult. Light-colored linen quickly washes out, acquires a yellow tint and loses its shine. The use of hydrogen peroxide to remove stains from fabrics is associated with some features:
- When restoring the material, do not mix dark and light underwear to avoid shedding.
- Old yellow stains from wool and silk are removed in several stages. Clothing is placed in a mixture of peroxide diluted with water. After 30 minutes, the product is drained, the laundry is washed and dried. If dirt remains, the procedure is carried out the required number of times until the color is restored.
- Removing yellow stains from cotton and linen is carried out by directly applying a peroxide solution to the material. If the yellowness remains, the procedure is repeated until the contamination disappears.
- Bleaching is carried out only once every 3-4 washes, otherwise the strength properties of the material will be lost.
- After bleaching, clothes are rinsed in 2 stages: first at a temperature of 20-40 ° C, and then in cold water.
Each type of matter has its own recipe for restoring whiteness. Therefore, when bleaching, things are separated not only by color, but also by type of fabric: synthetics, silk and wool, or cotton and linen.
How to protect the surface from yellowing
To prevent yellowing, regularly wash the plastic with soapy water and special products that create a protective layer. If possible, limit exposure of plastic to sunlight and heat. Do not smoke in the kitchen or indoors where there are plastic windows - they may turn yellow. Don’t despair if you can’t remove the old yellow stains on your windows - professionals can handle any stains. Order your apartment windows washed using high-quality cleaning products by experienced cleaners and enjoy cleanliness and comfort.
Cleaning medical gold
Medical gold is an alloy of brass, silver, copper, titanium and zinc, but there may be no gold in it at all: the alloy already has a noble “golden” luster. Products made from medical gold are durable, not subject to external mechanical influences, and due to the special technology of polishing and sputtering, they practically do not darken, do not lose shine, and do not change color.
- If it is necessary to clean such jewelry from contamination, the following methods are used:
- Rinse in a solution (2 drops of shampoo or liquid soap per 0.5 cup of water) and clean the product with a soft brush.
- To remove dust, wipe the product with a cotton swab soaked in beer; there is no need to rinse with water after this.
- Wipe the decoration with table vinegar, and then be sure to rinse in running water.
Using an Electrolyzer
This is done using a special electrolyzer device. It is a tube containing alkali. It also contains a pair of nickel electrodes. It is based on the principle of polarity. During operation, oxygen will be directed to that part of the pipe where the positively charged pole of the electrode is located, and hydrogen will tend in the opposite direction to the negative pole. This method of obtaining O2 and H2 is more suitable for laboratories. In addition, it is not designed for large volumes of gas production.
Bleaching of cellulosic materials
Textile fibers. Almost all cellulosic fibers, such as cotton, flax, jute or rayon, can be bleached with either chlorine derivatives or hydrogen peroxide (or both). Previously, calcium or sodium hypochlorite was usually used for this purpose, which was explained by the relatively high cost of hydrogen peroxide per unit of bleaching ability and little knowledge of methods for maintaining it in a sufficiently stable state in storage and during use. However, hypochlorite has been largely replaced by hydrogen peroxide as a result of improvements in techniques for stabilizing, transporting and storing peroxide, as well as through experience, which has shown the following:
- Hypochlorite is a more powerful oxidizing agent than hydrogen peroxide. True, it is possible to achieve the same degree of whiteness with minimal loss of tensile strength of the fiber using either one or another bleaching agent, but this requires more careful adherence to the production regime when using hypochlorite than when bleaching with hydrogen peroxide. In the latter case, there is less possibility of fiber destruction and a decrease in its strength due to the too strong action of the bleaching agent.
- The decomposition products of hydrogen peroxide are oxygen and water, and therefore the necessary processing after bleaching is simplified.
- The suitability of hydrogen peroxide for bleaching various types of fiber makes it possible to use the same equipment for different textile products.
- Hydrogen peroxide can be used to bleach yarn products dyed with vat or other dyes that may be damaged by chlorine bleaching. Cotton bleached with peroxide responds well to dyeing, is soft to the touch and has a more stable white color.
In some operations, hydrogen peroxide is used as an "antichlor" after hypochlorite bleaching. Products bleached with hypochlorite sometimes slowly turn yellow over time. This color change is apparently due to the formation of chloramines from hypochlorite and proteins contained in raw cotton fiber. Subsequent treatment with hydrogen peroxide prevents yellowing due to the decomposition of chloramines, and the weakening of the fiber from hydrogen peroxide is less than when using an antichlorine such as sodium thiosulfate. Depending on the processing conditions, hydrogen peroxide may also contribute a significant portion of the overall whitening effect.
The technique for bleaching cellulose fibers strongly depends on the nature of the material being processed, and for the bleaching of new fibrous substances, as a rule, this technique has to be developed by more or less empirical methods. There are literally hundreds of different recipes published for bleaching various substances; these recipes differ in such indicators as time, temperature, concentration, nature and amount of stabilizers, method of pre- and post-processing. Here are just a few typical techniques.
The most important characteristics of the bleached material that determine the type of subsequent processing are the cellulose content and the amount of lignin present. Pure cellulose is relatively inert to alkali without oxygen, but lignin and low-molecular-weight cellulose-type substances, such as hemicelluloses, are easily attacked by alkali. Thus, the permissible intensity of fiber processing depends on the relative content of lignin and other non-cellulosic substances, which act as cementing and strengthening substances for the fiber. Thus, cotton, which is a very pure form of cellulose, can be treated at high temperatures with caustic soda to remove foreign impurities, such as waxes, without reducing the strength of the fiber, and it can be bleached with hydrogen peroxide under relatively harsh conditions, for example, at pH values up to about 11 and at boiling temperatures. Slater and Richmond divide all plant fibers, except cotton, into 3 groups depending on the cellulose content and the permissible intensity of processing: 1) fibers containing over 85% cellulose and a small relative amount of lignin or pectic cementitious substances, such as flax; 2) fibers containing less than 85% cellulose and 6-18% lignin, such as jute, sisal or Phormium tenax (New Zealand flax); 3) fibers with an exceptionally high lignin content, such as coconut with 34% lignin.
Bleaching of fibrous substances containing appreciable quantities of lignin or other non-cellulosic substances is usually accomplished by treating first with hypochlorite and then with hydrogen peroxide. Below is a description of a typical flax yarn bleaching operation as an example of the treatment of Group 1 fibers. The yarn is first knitted for 3 hours. at 100° in a solution of sodium carbonate (instead of caustic soda used in cotton bobbing). This is followed by treatment with a dilute solution of calcium hypochlorite containing 5.6 g of active chlorine in 1 liter, acidification with 0.1-0.2% sulfuric acid and keeping at 71-77° for 4-5 hours. in a solution containing 0.1-0.2% hydrogen peroxide with the addition of sodium carbonate and sodium silicate. If linen yarn is bleached until completely white, the fiber noticeably loses its strength, and therefore partially bleached fabrics are often produced and sold.
In jute, a material belonging to group 2, the cellulose fibers are relatively short (2-5 mm) and are cemented together by lignin and hemicellulose, which, although they themselves have low tensile strength, provide the bulk of the mechanical strength of the fiber. Because they are more easily attacked than pulp, bleaching and other processing operations. should be produced under milder conditions than those used for cotton and materials of the 1st group. Typical processing of jute yarn consists of briefly soaking it in a solution of calcium hypochlorite containing 2.5-3.0 g of active chlorine and 0.7 g of sodium carbonate per liter, followed by bleaching with the same peroxide solution as indicated above, but in for 1.5-2 hours. Coconut fibers are also bleached in the same way, but in this case, preliminary treatment with bisulfite and final treatment with sodium hydrosulfite are required. Man-made fibers made from cellulose, such as rayon or silk acetate, require a relatively moderate bleaching regime. Silk acetate is especially susceptible to hydrolysis at high pH. A typical bleaching operation involves holding for 30-45 minutes. in a bath with 0.1-0.2% hydrogen peroxide with the necessary stabilizers added to it; the bath temperature should be maintained at 71-82°, pH within 8.8-9.5.
The bulk of hydrogen peroxide in the textile industry is used to bleach cotton. Cotton can be processed in a variety of forms, such as yarn, lump or finished goods in batch-type operations, or in tow or spread form in a continuous bleaching line. Batch operations are usually carried out in cement-lined iron kettles (boilers) or in vats or tanks made primarily of stainless steel. Ceramic tile cladding may crack at high bleaching temperatures. The initial processing of cotton may vary, but in all cases a thorough preliminary cleaning is required. A typical hydrogen peroxide bleach bath contains 0.1-0.4 wt. % of the latter, about 1-1.5% sodium silicate and a sufficient amount of sodium hydroxide to bring the pH to 10.5-11.5. For periodic operations, the tissue is treated with this solution for 2-8 hours. at 80-100°, usually using some type of stirring. To bleach goods dyed with naphthol dyes, a bath is used, the pH of which is maintained within the range of 7.0-9.0 to avoid dye decomposition.
Most cotton is processed using the continuous process. In a typical installation of this type, cleaned cotton goods are soaked in a 3-4% solution of sodium hydroxide and then continuously passed through the top of a shallow J-shaped box, in which the fabric is kept for 1-1.5 hours and steamed at 100°. The tissue is continuously removed from the bottom of the J-box, washed, soaked in a bleach solution containing about 0.2-0.5% hydrogen peroxide, 1-1.5% sodium silicate and 0.05-0.25% sodium hydroxide to creating a pH of about 10.5, and then steaming for about an hour at 99-100° in a second J-box. The tissue is reported to move at speeds of 73–275 m/min.
The role of sodium silicate, which is usually added to the bleaching solution, is multifaceted. It acts as a stabilizer, reducing wasteful loss of hydrogen peroxide due to decomposition, as well as a buffer to help maintain pH at the desired level. At the same time, it has a certain cleaning and impregnating ability and anti-corrosion properties in relation to metal equipment. Wetting and impregnating agents can also be added to the bleach solution. The optimum pH is determined by a compromise between two extremes: at high pH (above about 11.5) rapid bleaching occurs, but the hydrogen peroxide also decomposes quickly, and very careful control is also required to ensure proper bleaching without compromising fiber strength. At significantly lower pH values, the bleaching rate becomes so slow that the process becomes uneconomical. To bleach 1 ton of cotton, approximately 12 kg of a 27.5% solution of hydrogen peroxide and 5 kg of sodium silicate are required [12].
Synthetic fibers, such as viscose silk, nylon, orlon (polyacrylonitrile), etc., are generally of satisfactory whiteness during production. And if bleaching turns out to be necessary, it is usually only to remove stains or yellowing that arise when processing the yarn before knitting or weaving or when widening the fabric to ensure the proper size. Viscose silk is usually bleached using methods similar to those used for cotton. For acetate fiber, a neutral or slightly acidic bath must be used to avoid hydrolysis. A bath containing peroxoacetic acid neutralized with sodium hydroxide and some polyphosphate is often used. Peroxoacetic acid is also successfully used to bleach nylon. Hydrogen peroxide does not appear to have any effect on Orlon, Dynel (a copolymer of vinyl chloride and acrylonitrile) and acrylan (a copolymer of vinyl acetate and acrylonitrile).
Recently, a valuable bibliography was published by a commission of the American Association of Textile Chemists and Colorists [13]. It provides brief abstracts of all articles on textile bleaching published during the period 1900–1950. This bibliography contains 245 references to works on bleaching with peroxide, 153 to works on bleaching with hypochlorite (a significant part of them relate to bleaching with both of these substances simultaneously) and 321 to works on the use of other chemical agents for bleaching, equipment, etc. e. Additional detailed information on the technique of bleaching cellulosic textiles can be obtained from these works, and can also be obtained from various companies that produce hydrogen peroxide, or read in books on cellulose and cotton. One of Bell and Stalter's reports examines the effect of various properties of cotton pieces on the continuous hydrogen peroxide bleaching process. Quantitative data are provided showing how this changes whiteness, fluidity, absorbency, oil, fat, wax and ash content, as well as pH.
The bleaching effect of hydrogen peroxide is also used to remove stains and bleach laundry during home washing. A three percent solution of hydrogen peroxide is diluted in a ratio of 20: 1 with lukewarm soapy or clear water containing a small amount of ammonia and kept. Hydrogen peroxide soak the laundry in this water for 30 minutes, then rinse and dry; Thus, the benefits of bleaching with hydrogen peroxide are also used at home. Due to the difference in price and the need to use two liquids, the possibility of competition between hydrogen peroxide and hypochlorite for bleaching at home is eliminated; however, by using powdered bleaching agents based on sodium peroxoborate, the noted disadvantages are eliminated, and these substances, despite their increased cost, find significant use. From the point of view of market expansion, it is interesting that this type of bleaching baths has been popular in Europe for a long time, but only recently began to spread in the United States. The reason seems to be that the bleaching action of such a peroxyhydrate requires a slightly higher temperature than usual; In Europe, as far as is known, boiling washing is widely practiced, and therefore high temperatures are already used, while in the USA washing at the required high temperatures has only begun to spread due to the recent advent of household automatic washing machines.
Bleaching of wood pulp. Wood contains about 45-55% cellulose, with the rest consisting of carbohydrates (for example, pentosans and hemicelluloses) and non-carbohydrates (for example, resins, fats, waxes, lignin, tannins and ash).
Paper and paper products are produced from wood products obtained in two ways. Wood pulp is obtained by debarking and subsequent mechanical grinding of wood and contains almost all the constituent parts present in the tree. Paper containing wood pulp quickly deteriorates, especially when exposed to sunlight and air, since it contains lignin and other relatively unstable substances. Chemical pulp can produce significantly more resistant paper, since in this case almost all non-carbohydrate components of the wood are removed during the cooking process and subsequent bleaching operations. In some quantities (through less complete processing) products of intermediate composition are also produced, known as semi-chemical cellulose.
There are three chemical processes for removing lignin from wood:
- The sulfite process is based on the use of a hot aqueous solution of sulfur dioxide in combination with calcium or magnesium bisulfite. Typically, this solution is used for cooking softwood, such as spruce and hemlock.
- The soda process, which is currently of less importance and is based on the use of caustic soda solution; as a rule, it is used for boiling deciduous wood, such as poplar.
- The sulfate or kraft process uses a solution of sodium hydroxide and sodium sulfide. Typically, this method is used to boil pine and similar woods with a high resin content, although this method is suitable for any type of wood. The alkaline cooking liquid is weaker than the cooking acid used in the sulfite process; therefore, kraft pulp has higher strength but also contains higher amounts of non-cellulosic wood components. In this regard, such cellulose, if achieving high whiteness is desired, requires more intensive bleaching.
In any of these three chemical pulping processes, it is desirable to remove as much lignin and similar substances as possible while minimizing the destruction of cellulose molecules. This is best achieved by stopping the cooking process at a stage when no non-cellulosic substances have been removed, followed by cleaning and bleaching of the resulting cellulose. The purpose of this post-treatment is to decompose the colored substances retained in the fiber and remove non-cellulosic substances. Typically the process is carried out using one of the following two methods: 1. Cellulose is treated with a solution of calcium hypochlorite or sodium hypochlorite (which is obtained by reacting a calcium salt with sodium carbonate or sodium hydroxide) with a pH of 8 to 11 in the presence of cellulose; under these conditions, cellulose is obtained, colored creamy. 2. In the so-called two-stage or multi-stage method, the cellulose is treated with an aqueous solution of chlorine (chlorine reacts with water to disproportionate to hypochlorous and hydrochloric acids and gives a pH below 2). After this, the mass is neutralized with calcium hydroxide (or sodium hydroxide), which extracts the substances that went into solution during treatment with chlorine. The cellulose is then treated with hypochlorite or peroxide. Treatment with chlorine and washing with alkali reduces the color intensity relatively little and is usually considered as one of the cleaning stages. But after such preliminary cleaning, subsequent bleaching is especially effective. If proper whiteness is desired, the alternating chlorine and alkali treatment can be repeated two or three times before the bleaching stage. The intensity of bleaching and its type depend on the distinctive characteristics of the original wood, the cooking process and the final use of the pulp.
Many types of paper are produced from mixtures of wood pulp and chemical pulp so as to obtain at a minimum cost the proper combination of whiteness, fastness, printability, opacity, mechanical strength, absorbency, etc., according to the intended application. For example, the pulp used for newsprint, where low cost is the most basic requirement, consists predominantly of wood pulp; For high-quality paper (for books), only mixtures of chemical types of cellulose are used. In the production of various papers for short-term use, such as for magazines and catalogs, as well as in the production of wrapping, tissue and filter papers, mixtures of chemical pulp and wood pulp are used. All such materials require significantly more intensive cleaning and more bleaching agents per unit weight of material processed than textiles. Since the chemical cost of using hydrogen peroxide or sodium peroxide is greater than the cost of hypochlorite-based bleach solutions, peroxides are economical only when the quality of the final product is so significantly superior to that achieved with hypochlorite that it offsets the higher costs.
The almost infinite number of options regarding the types of raw materials and the desired properties of the final product is the reason that a wide variety of bleaching processes are applicable under different conditions; this also contributes to the fact that in the competition of peroxide with chlorine, hypochlorite and other agents, peroxide is not in such a favorable position as, for example, when bleaching cotton. It is obvious, however, that peroxides are of particular importance in the bleaching of wood pulp. Hypochlorite is usually not used for this purpose, since it must be used in such a high concentration that it can lead to a significant decrease in the yield of the final mass. Treatment with peroxide can increase the whiteness of wood pulp by approximately 10-12 G. E. units with a negligible decrease in pulp yield. This makes it possible to use a significant amount of such cheap pulp in a mixture intended to produce printing or tissue paper of satisfactory whiteness. Along with its low cost, wood pulp has certain advantages, such as greater opacity (allowing the use of lighter paper) and some desirable printing properties (resilience). However, it must be borne in mind that this kind of bleaching does not significantly increase the low resistance of wood pulp to sunlight.
In 1950, wood pulp was bleached with hydrogen peroxide or sodium peroxide at 24 plants with a total production capacity of 1130 tons per day, while mixtures of wood pulp and sulfite pulp were bleached with peroxides at a number of plants with a total production capacity of about 360 tons of printing paper per day. For this purpose, you can use both hydrogen peroxide and sodium peroxide or a mixture of both of these substances. At market prices in 1954, sodium peroxide was approximately 25% cheaper than hydrogen peroxide per mole of peroxide ions. To ensure the proper pH of the bleach bath, the sodium peroxide must be partially neutralized with an acid such as sulfuric acid; on the other hand, it is usually necessary to add sodium hydroxide to hydrogen peroxide. Thus, when using an appropriate mixture of both peroxides, there is no need to use any additional chemicals other than stabilizers.
The most economical use of peroxides for bleaching wood pulp is in systems with high consistency, that is, with a relatively high ratio of mass to bleaching solution. The desired alkalinity after introducing wood pulp into such a bath corresponds to a pH of 10.0 to 10.5; this pH drops as the bleaching process progresses. Bleaching time depends on the consistency of the mass, temperature, alkalinity, peroxide content and desired whiteness. A typical regime is based on the use of a 12% consistency of the mass, hydrogen peroxide in an amount of about 1% based on the dry weight of the mass with the addition of sodium silicate and magnesium sulfate (or one of them) at a temperature of 38-43°. After a two-hour treatment, the brightness level increases by approximately 12 G. E. Peroxide bleaching can be carried out in a batch or continuous manner in the same equipment used for hypochlorite bleaching.
Another technique is to apply a peroxide solution to paper or cellulose and leave it at room temperature for one or more days. Bleaching can be carried out, for example, during storage or transport. In addition to the usual precautions taken to prevent peroxide decomposition, wood pulp bleaching processes require careful control of bacterial growth through the use of suitable inhibitors, as many of these bacteria produce the enzyme catalase, which actively decomposes hydrogen peroxide. Sodium hydrosulfite Na2S204 or zinc hydrosulfite is also used for bleaching wood pulp; Hydrosulfite is often used in series with hydrogen peroxide.
Sulfite cellulose at various plants is also subjected to bleaching with peroxides in combination with hypochlorites, but in this area peroxide has not yet found significant technical application. Most often it is currently used for the production of special grades of paper, which require an increased degree of whiteness. Hydrogen peroxide bleaching can only be economical as a final processing step after the lower-cost preliminary processes of chlorine cleaning and hypochlorite oxidation (or one of these). The main advantages of this method over the hypochlorite process are significantly increased whiteness and color stability, achieved without noticeable destruction, which justifies the higher cost. Treatment with peroxide also allows for higher pulp yields in bleaching processes and increases the heat resistance of bleached pulp, which is of significant importance in further papermaking operations. Peroxide solution differs from other bleaches in that it bleaches lignin and other colored substances, but does not dissolve them to a noticeable extent. Thus, sulfite pulp can be bleached with hydrogen peroxide in one operation to obtain a very high pulp yield, but the cost of chemicals in this case is so significant that the process is not economically justified. To bleach kraft pulp, peroxides can be used together with hypochlorite. In this case, sodium peroxide can be used at the alkali extraction stage. When used in this way, sodium peroxide is cheaper than hydrogen peroxide.
In the final bleaching of chemical pulp, hydrogen peroxide can be competed with chlorine dioxide gas, which imparts a very high degree of whiteness to the pulp with a minimal decrease in strength. The disadvantages of this method are the high cost of chlorine dioxide, its corrosive properties, as well as toxicity and explosiveness at concentrations exceeding a known limit. Chlorine dioxide, usually produced outside the bleaching plant from sodium chlorate, is absorbed into water.
Cleaning paper from printing inks. By combining mechanical and chemical deinking processes, waste paper can be processed so that the resulting pulp can be used for new production of printing or other light-colored paper. Typically, hot solutions of sodium hydroxide or sodium carbonate or mixtures of both are used for this purpose. They react with various binders in the paper, especially the ink binder, and thereby help separate the fiber from other impurities present. Alkaline peroxide solutions are more effective than caustic alkalis with the same pH, and although the cost of chemicals in the first case is higher, peroxide makes it possible to process lower-quality waste paper, carry out cooking at lower temperatures and obtain higher yields of paper pulp and a cleaner product. In addition to the fact that peroxide has a bleaching effect, it also functions as a substance that accelerates the hydrolysis and depolymerization of proteins, starches and oils, which are components of adhesives and printing ink binders, and therefore the pigments become soluble and come off the paper. As with other types of applications, it is permissible to use hydrogen peroxide, sodium peroxide, and a mixture of them with adjustment of the required pH and the addition of stabilizers. The use of peroxides is especially desirable for removing paint from paper containing wood pulp, since such paper darkens noticeably with normal alkaline treatment.
Bleaching of other cellulosic materials.
Other cellulosic materials can also be bleached with peroxides using equipment similar to that used for bleaching cellulose fibers and wood pulp. Straw bleaching is a long and difficult operation; it must be produced under mild conditions by prolonged and repeated alternating soaking in baths of peroxide and a reducing agent, such as sulfur dioxide or sodium hydrosulfite. Hydrogen peroxide is especially useful for bleaching wood used, for example, in light-colored furniture, radio and television cases, and baseball bats. According to one frequently used method, a 5% solution of caustic soda containing sodium silicate is applied to the surface of the wood with a brush or spray, and after some exposure it is covered with a 27-35% solution of hydrogen peroxide; these operations can be carried out in reverse order. After reaching the intended whiteness, the alkali is neutralized with weak acetic acid and the wood is dried. Instead, the wood can be bleached in a one-step operation using a mixture of peroxide, sodium silicate and ammonia or caustic soda. As a result, only a thin surface layer is bleached, and therefore subsequent finishing operations require careful and precise execution. Tags: bleaching
Reviews
Good afternoon everyone! The other day I read on the Internet how to whiten your teeth and remove plaque, stones and all that at home in one session. In short, take half a teaspoon of soda, pour 15-20 drops of hydrogen peroxide into it and dip it into this mixture with a cotton swab and... rub it into your teeth. For better effect, add lemon juice. (this is for better breath smell) Read and done! I scrolled through everything as written. I rubbed the cotton wool on my fangs and then I felt the gag reflex begin! Hydrogen peroxide turned out to be so disgusting in taste, and even together with soda - complete bummer! I quickly rinsed my mouth and brushed my teeth with normal paste - everything seemed to go away. And in the morning I look, and my teeth are really like snow! So I advise you to try everything!
Maksim
It’s better not to experiment with your own teeth - baking soda and simple peroxide won’t do anything good to your teeth. For safer whitening, special formulations are created that contain stabilizers, medicinal substances and the actual active components based on peroxides. – science does not stand still – but strives for safe medicine!
Director
Do not whiten your teeth with peroxide and soda under any circumstances; it is better to buy a professional product or go to the dentist! A friend of mine decided to whiten her teeth on her own using baking soda and peroxide... After this procedure, tooth enamel became thin and her teeth were sensitive. Better yet, just eat strawberries
AnnaLee
Hydrogen peroxide and baking soda don't help me. Still, it’s better to go to professionals.
Mar5
I remove yellow plaque from my teeth with regular hydrogen peroxide.
MamaKati
zubneboley.ru
Why does plastic turn yellow?
Plastic can turn yellow due to improper care, sudden temperature changes and low-quality additives in the polymer, and fade in the sun. Let's look at the five main reasons why plastic turns yellow.
Burns out in the sun
Ultraviolet radiation destroys the polymer compounds of plastic. To avoid yellowing, light stabilizers and benzophenol are added to plastic during production, which block the penetration of sunlight into the plastic and color change.
Temperature changes
With sudden temperature changes, the plastic is deformed and microcracks appear in it, into which air begins to flow. This leads to slow oxidation of the top layer of plastic and yellowing.
Improper care
The surface layer of plastic can be destroyed by acids, alkalis, aggressive cleaning agents and hard brushes. At the same time, microcracks form on the surface of the polymer, into which dirt gets trapped, changing the color of the material.
Dust, grease and soot
If there is no hood in the kitchen or it cannot cope with the outflow of air, and people smoke at home, this leads to soot, dust, grease and nicotine deposits settling on plastic items. They penetrate the surface layer of the plastic and change its color. First of all, plastic windows in the kitchen turn yellow.
Poor quality material
Violating plastic production technology, using recycled materials or saving on color additives worsens the surface and protective layer of the material. This leads to the rapid appearance of yellow spots on the surface.
For what indications is it used?
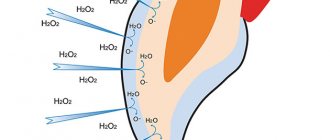
Enamel whitening with peroxide is usually achieved for the following indications:
- When teeth change and darken during natural age-related changes;
- If the darkening of the enamel occurs due to drinking coffee and other drinks that contain coloring substances, as well as smoking;
- Yellowing of the enamel with increased use of toothpastes with a high fluoride content, which are intended for the prevention of carious lesions;
- For yellowing of enamel due to increased use of tetracycline.