1855
Technologists have thoroughly studied the physical properties of medical plastics, which has made it possible to widely introduce fast-hardening material into the practice of dentistry, orthodontics and maxillofacial surgery.
Orthodontic appliances are designed to correct abnormalities of the jaw system. However, the design of the devices will not function properly without fast-hardening materials.
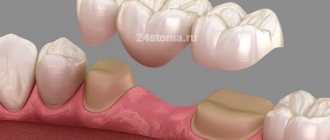
In addition, acrylic substances are used to make dental crowns used in prosthetics.
General overview
Rapid-hardening plastics are also called self-hardening. In medical practice, the adaptation of this type of material has technologically changed the process of creating structures.
Peculiarities:
- the ability of the material to undergo accelerated polymerization at everyday room temperature, or human body temperature;
- high level of compatibility of the material with other forms of substances;
- there is no destructive effect of the material on the oral mucosa.
In addition, medical compounds are used to repair damaged prosthesis bases.
Also, materials of this type make it possible to restore a combined non-removable structure directly in the oral cavity .
Classification of polymers
Polymers used in prosthetic dentistry belong to one of three groups. Each of them is subject to different hygienic, toxicological, aesthetic, and technological requirements.
- Basic, or basic polymers in dentistry are used for the manufacture of artificial teeth and bases for removable dentures.
- Auxiliary - needed for impressions, molding and modeling.
- Clinical polymers include sealants, restoratives, and adhesives.
Composition and properties
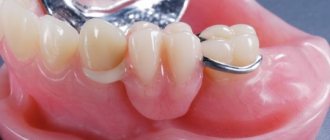
Quick-hardening plastic masses are used in orthodontics in various variations, most often in the manufacture of removable and non-removable appliances and their parts.
Each factory package contains a powdery substance (varieties of acrylic), liquid (methyl methacrylate with various additives) and activators.
When these three components are combined, a solid dental material for prosthetics is formed, and polymerization occurs.
Functional features of the constituent elements:
- Powder is an ether polymer of methacrylic and acrylic acids with various metal additives. This substance also contains dyes and plasticizers. The powdery substance is the basis for creating materials.
- The liquid consists of ether compounds of methacrylic acid, chemical compounds similar in composition - monomers and various additives.
- Monomer is an ethereal transparent consistency with a strong characteristic odor.
Improper storage can cause self-polymerization, so it is important to tightly seal the bottle and add an inhibitor to prevent the substance from hardening. The function of this substance is to polymerize the composition without heating. - Fillers are able to add viscosity to the powder, increase the strength of the composition and protect polymers from rapid wear. In addition, there are antimicrobial fillers that prevent the formation of microorganisms in polymers.
High quality medical plastics must be packaged in kits containing liquid and powder.
What design of a removable denture for the upper jaw do dentists consider the best?
Come here if you are interested in the reasons for the development of denture stomatitis.
At this address https://www.vash-dentist.ru/protezirovanie/semnyie-p/vidov-pri-otsutstvii-bolshogo-kolichestva-zubov.html we will consider the types of prosthetics in the absence of a large number of teeth.
Water resistance and water absorption of polymers
Water resistance is the ability of polymers to retain their properties under prolonged exposure to water. If water gets inside the polymer, it swells, its shape is distorted, and its strength indicators suffer. Moisture resistance is resistance to humid air. Due to absorption, water vapor also causes swelling of hydrophilic materials. However, more often moisture accumulates in the surface layer due to adsorption, penetrating into microcracks.
The water resistance of the polymer is characterized by water absorption. This parameter indicates the amount of water that the material is able to absorb when kept at a temperature of 18-22 ° C for 23 hours. Due to water absorption, the geometric shape of the prosthesis base changes, and the mechanical properties deteriorate. The higher the water absorption, the more susceptible the polymer is to the penetration of microorganisms.
The presence of sorption water in the polymer sharply reduces its strength, hardness, rigidity, and indentation resistance. The polymer loses soluble substances, so its properties change.
Advantages and disadvantages
The materials are used in medicine for various purposes. Versatility is associated with a set of good basic physical and chemical properties.
The advantages of the material in orthodontics are undeniable:
- do not have harmful effects on the body;
- the material is highly durable and can withstand strong mechanical stress;
- the ability to eliminate the breakdown of a fixed prosthesis directly in the oral cavity;
- the ability of the material to quickly self-polish (harden) under normal conditions, which simplifies the process of filling teeth;
- has a color similar to the natural color of the dental crown;
- a fast, simplified material processing technique that reduces the time required for manipulations in the patient’s oral cavity.
However, there are also disadvantages:
- inexpensive types may have an unaesthetic appearance;
- may darken during prolonged use;
If the consistency is not prepared correctly, the material becomes insufficiently strong and is susceptible to chipping.
Plastic tires
For jaw fractures combined with radiation injuries, the use of metal splints is contraindicated, since metals can become a source of secondary radiation, causing necrosis of the gum mucosa. It is more expedient to make tires from plastic. M.R. Marey recommends using nylon threads instead of ligature wire to secure the splint, and making the splint for fractures of the lower jaw from fast-hardening plastic AST-2 along a pre-made aluminum groove of an arcuate shape, which is filled with freshly prepared plastic, placing it on the vestibular surface of the dental arch. After the plastic hardens, the aluminum gutter is easily removed, and the plastic is firmly connected to the nylon threads and fixes the jaw fragments.
The method of applying a plastic splint was modified in the clinic of prof. G. A. Vasilyeva. A nylon thread is applied to each tooth, with a plastic bead on the vestibular surface of the tooth. This creates a more reliable fixation of the ligatures in the splint. Then a splint is applied according to the method described by M.R. Marey. If intermaxillary fixation of jaw fragments is necessary, it is recommended to drill holes in the appropriate areas with a spherical bur and insert pre-prepared plastic spikes into them, which are fixed with freshly prepared quick-hardening plastic (Fig. 312). The spikes serve as a place for applying rubber rings for intermaxillary traction and fixation of jaw fragments.
R. M. Frigof proposes the following method for making a plastic splint for fixing fragments of the lower jaw. This technique is applicable in cases of linear fractures along the dental arch in the presence of teeth on both fragments and the possibility of their manual reduction. After setting the jaw fragments into the correct position, closing the teeth, they are secured using a ligature bandage, which should cover 2 teeth from the fracture line on the vestibular side and 2 adjacent teeth on the lingual side (first ligature) (Fig. 313). The ends of the wire are brought out through the interdental spaces of adjacent teeth. The second ligature wire covers 2 teeth located on the fracture line on the lingual side. The ends of both ligatures are twisted, cut to a length of 1 cm and spread apart. Then quickly hardening plastic is kneaded, the dough is formed into a roll 1 cm thick and 4 cm long and applied to the chewing or cutting surfaces of the teeth on the sides of the fracture line (covering 3 teeth on each side). The teeth on the vestibular and oral sides are pressed with plastic, after which the patient closes his mouth in the position of central closure of the dentition and keeps the jaws closed for 4-5 minutes until the plastic hardens. To eliminate the possibility of burns to the mucous membrane and oral cavity, the plastic block is covered with cotton swabs moistened with cold water. The advantage of this method is good fixation of jaw fragments and the possibility of free movements in the temporomandibular joints.
Source
Popular products
Several basic types of plastics, which are universal, are widely used in dental practice.
The powdered composition should be mixed in certain proportions, and it can be used in different viscosity states.
Protacryl
The substance is classified as a basic type of self-hardening plastic. Supporting properties are distinguished:
- powder with a pink tint contains polymethyl methacrylates, initiators, activators, opacifiers, dyes;
- manufacturability and high strength;
- does not change color upon contact with oral tissues;
- used in the reconstruction of removable dentures;
- in case of loss of adhesiveness, it is used for restoration of bases.
In addition, the material is widely used for the manufacture of various orthodontic appliances.
Noracryl
This product is most often used in medical practice to restore damaged bridges.
Characteristics of Noracryl:
- contains three types of powders, two liquid catalysts and a liquid solvent, a filler that reduces water absorption;
- the resulting mass is used for filling dental canals and applied to the dental cavity;
- The curing time of the material is minimal and reaches 5-7 minutes.
The duration of mixing this type of material should not exceed one minute.
Carboplast
A polymeric substance with a yellow color. Characteristics of carboplast substance:
- the composition contains a ternary copolymer of methyl acrylate, butyl methacrylate, liquid, epoxy resin, stabilizer, filler - chalk;
- the material kneads well in the hands after mixing, this allows it to be evenly distributed over the plaster model;
- hardens at room temperature directly on the model.
Widely used when creating casts and models for further orthopedic work.
Redont
One of the forms produced for medical purposes in three main versions - opaque Redont, unpainted and transparent Redont-02, pink transparent Redont-03.
Characteristics:
- The product consists of powdered copolymer of methyl methacrylate and ethyl methacrylate, liquid methyl methacrylate, inhibitor, activator;
- used to restore broken dentures;
- used in the design of various medical mechanisms.
The main use of Redont is to move removable dentures in cases where the fit to the tissues of the oral cavity is disrupted or when there is a loss of adhesion to dental crowns.
Stadont
Acrylic substance having the following characteristics:
- main composition: methyl copolymer powder, opacifier and liquid methacrylic acid methyl ester, catalyst, stabilizer;
- factory markings contain substances of three different shades;
- are used in the production of medical fixing splints used in the presence of periodontal disease.
The main purpose of the product is to use it for the repair of bridge structures and removable plate prostheses.
Acrylic oxide
The product is white in color and has positive chemical characteristics:
- main composition - resins (acrylic and epoxy), mineral fillers;
- the product is not subject to shrinkage;
- has a color similar to the natural color of teeth;
- has high plasticity.
It is used for filling canals, making crowns, modeling dental inlays.
Carbondent
An acrylic substance having a composition with enhanced physical and chemical properties. Characteristics:
- the powder contains methyl acrylate ternary copolymer, butyl methacrylate, liquid with the addition of epoxy resin, mineral fillers, a stabilizer, a substance that protects the material from aging;
- The preparation kit contains 6 powders, differing in color type;
- improved characteristics of strength and adhesiveness.
A mass prepared from powder is used to create dental impressions.
Five popular models of containers for dentures and their features.
In this publication we will tell you what compensation pensioners are entitled to for dental prosthetics.
Here https://www.vash-dentist.ru/protezirovanie/semnyie-p/nedorogie-zubnyie-nadezhnyimi.html read about the types of inexpensive dentures.
Cold curing plastics
Self-curing acrylic plastics are compounds that cure spontaneously at room temperature. The polymerizate, depending on the composition of the compound, can be hard or elastic. Self-hardening plastics are widely used in dentistry for correcting (relining) dentures, repairing dentures, making temporary dentures, splints for periodontal disease2, models, and individual impression trays. Self-hardening plastics have gained a strong place as filling materials. Cold-curing plastics have a number of advantages over hot-curing plastics, but are inferior to them in some respects. The technology for processing self-hardening plastics is simpler, no heating equipment is required, there is less change in product dimensions, less residual stress in products, and repair of the prosthesis can be done quickly in the presence of the patient. In some cases, self-curing materials cannot be replaced by hot-curing plastics. At the same time, self-hardening plastics are inferior in strength and contain a larger amount of residual monomer. Thus, hot- and cold-curing plastics do not exclude one another, but complement each other. The technology for the production of cold-curing plastics differs from the production of hot-curing plastics in that during the synthesis an initiator is introduced into the polymer powder in an amount of 1.5%, and an activator is added to the liquid.
Compound . Powder is a suspension homo- or copolymer, colored and turbid and containing a component of the redox system - usually an initiator.
Liquids of self-hardening plastics have the following composition:
- 1) linear polymers (monomer or mixture of monomers, OBC activator, inhibitor);
- 2) polymers of three-dimensional structure (monomer or mixture of monomers, OBC activator, cross-linking agent, inhibitor).
The production of dental structures from polymer-monomer self-hardening materials proceeds according to the following scheme:
Properties . Self-curing of acrylic compounds used in dentistry is due to the initiating action of the redox system (ORS). The main components of OBC are the initiator and activator. Organic peroxide can be used as an initiator. Benzoyl peroxide is usually used. Various compounds are used as activators: tertiary amines (primary and secondary ones inhibit the polymerization process), mercaptans, sulfinic acid derivatives, ascorbic acid, etc. In addition to the initiator and activator, some OBCs also contain promoters.
Let us consider the mechanism of action of cold-curing OBC. The radicals that initiate the polymerization process are formed during the decomposition of benzoyl peroxide. As can be seen from the kinetic curves of the decomposition of benzoyl peroxide obtained at different temperatures, the rate of decomposition depends on temperature and begins to decrease noticeably from the moment 65-75% conversion is reached (Fig. 19). For the effective initiating action of benzoyl peroxide, heating to a temperature above 65 ° C is required, at which vigorous decomposition of the peroxide begins. The activator reduces the activation energy for the decomposition of benzoyl peroxide, which is equal to 126 kJ/mol, and the decomposition of the peroxide begins at room temperature. OBC is the most important criterion for the quality of self-hardening plastics. This system should:
- 1) ensure complete monomer conversion;
- 2) do not cause changes in the color of the polymerizate under the influence of solar radiation and endogenous processes;
- 3) not be toxic;
- 4) be stable;
- 5) initiate the polymerization process at minimal concentrations;
- 6) provide the necessary working hours.
To avoid premature polymerization, the activator is usually introduced into the liquid, and the initiator into the powder.
O B C peroxide-amine type. The great practical importance of self-hardening plastics has stimulated a wide scope of research on the creation of cold-polymerized alloys.
Powder-liquid plastics
Tertiary amines were first proposed as activators (dimethylaniline) for cold polymerization in 1943 by Schvebel and Tromdorf. Based on this activator, the first self-hardening plastics AST-1, AST-2, AST-2A and (styracryl) were produced in the USSR (1952). It soon turned out that the use of dimepilaniline and other tertiary amines leads to a change in the color of the polymer. This occurs as a result of endogenous processes , in which the amine is involved. Strubell found that the color and light fastness of the plastic depends on the nature of the tertiary amine. Table 11 shows the effect of the nature of some amines on color fastness.
In the homologous series of dimethylaniline, color fastness increases from dimethylaniline to dimethylamino-p-isopropylbenzene. The most widely used are dimethylparatoluidine CH3•C6H4•N(CH3)2 and N-bis(2-oxyethyl)paratoluidine CH3•C6H4•N(OC2H5)2, which, having a high activating efficiency, are quite suitable for the production of self-hardening plastics used for repairs prostheses, relocation, manufacturing of orthodontic appliances and other works. The color fastness of plastics depends on the purity of the activator. Even the presence of traces of undermethylated products in dimethylparatoluidine (DMPT) causes a decrease in the color fastness of the polymer.
A technology has been developed for the synthesis of DMPT, free from traces of impurities that cause coloration of the polymer product. The completeness of monomer conversion depends on the efficiency of the OBC and the temperature of the polymerizing molding mass. Rice. 20 illustrates the dependence of polymer temperature on time when using some amines. The most effective is dimethylaniline. However, in terms of its set of properties, DMPT can be considered optimal, while compounds based on dimethylaniline are used only for technical needs. In order to increase color fastness, work was carried out to stabilize color fastness. Foreign information about the stabilizing effect of carboxylic acids and their anhydrides has not been confirmed. An increase in color stability was achieved by introducing up to 1% 2-oxide-5-methylphenyl-benztriazole.
OBC based on sulfonic acid and its derivatives. Tertiary amines are very effective activators, but they still do not provide long-term color stability under light radiation conditions. In addition, it is desirable to have OBCs that make it possible to obtain a polymer product with a higher degree of conversion. The search for new activators led to the discovery of the activating effect of sulfinic acids, as reported by Hagger in 1948. Sulfinic acids make it possible to obtain color-stable polymers, but their low chemical resistance and lower activity compared to amines have limited their use. Due to its easy oxidation by air oxygen, sulfinic acid was mixed with silicone oil and stored in a tube in the form of a paste. Before preparing the molding mass, the required amount of paste was squeezed out and squeezed out with filter paper. Sulfinic acid crystals were dissolved in the monomer before mixing it with the powder. In paste form, sulfinic acid can be stored for 6 months.
M. M. Gerner, L. N. Mats proposed OBC based on stable derivatives of sulfinic acid. In addition to residual benzoyl peroxide, stable sodium sulfate is added to the powder, and a small amount of methacrylic acid is added to the monomer. Thus, in this case, the activator and peroxide are contained in the powder. When mixing the powder with the monomer, the reaction occurs: CH2=C(CH3) • COOH + C6H5SO2Na → CH2=C(CH3) • COONa + C6H5SO2H.
Active sulfinic acid C6H5-SO2H forms with benzoyl peroxide an OHS of cold polymerization, and an excess of methacrylic acid and the resulting sodium salt participate in copolymerization with the ether. The introduction of methacrylic acid increases the adhesion of the polymer due to carboxyl groups to various substrates. This OBC is used in the self-hardening plastic noracryl.
OBC based on sulfones. Research by Brederice et al. showed that α-hydroxy and α-aminosulfones can be successfully used as effective polymerization activators, which give more color-resistant polymers. Their effective use is only possible in combination with cocatalysts, monohydric alcohols, compounds of metals of variable valence (Сu2Сl2), etc. Good results can be obtained using N-ethyl-bis-(p-tolylsulfonmethyl) amine (CH3•C6H4•SO2CH2)2• N•C2H5 and N-methyl-bis-(p-tolylsulfonemethyl)amine (p—CH3C6H4SO2CH2)2•N•CH3. Sulfonamines at room temperature without cocatalysts practically do not accelerate the polymerization process. At 40°C, polymerization occurs in 22–26 minutes with a temperature peak of 82–84°C. With the introduction of only 0.01% Cu2Cl2, curing occurs in 7–11 minutes with a temperature peak of 95 °C.
Copper chloride I is introduced into the monomer, and since it is insoluble, the liquid must be shaken before mixing with the powder. When 8% methanol is introduced into the liquid, curing occurs at room temperature. Three-component OBC (1.5% benzoyl peroxide, 8% methanol and 0.01% copper I chloride) provides curing in 5-7 minutes. In Fig. Figure 20 shows curves of the temperature of the polymerizing molding mass versus time for various compositions of sulfone-based OMS. The introduction of a small amount of DMPT up to 1%, which has virtually no effect on color fastness, significantly increases the conversion of the monomer, since the temperature peak reaches 94-98 °C. Cold polymerization does not lead to complete monomer conversion. After polymerization is completed, the polymer contains 3-5% residual monomer during cold polymerization and 0.5% during hot polymerization.
OBC based on mercaptans. Peroxide-mercaptan type OBC is widely used for the vulcanization of rubbers and can be used to cure dental acrylic compounds at room temperature. Self-hardening plastic Orthofil (England) contains OBC of the peroxide-mercaptan type. In the reaction between peroxide and mercaptan, the latter plays the role of a reducing agent.
To create acrylic compounds in dentistry, lauryl mercaptan C12H25SH (synonym: dodecyl mercaptan) is used as an activator. The advantage of these OBCs is the color fastness of the polymer. The OVS currently used cannot be considered perfect. The search for new systems is carried out in two main directions - increasing color stability and increasing monomer conversion.
Preparation of molding mass . The technology for preparing the molding mass of self-hardening plastics is identical to that described. From each batch you can only manage to mold one product. During polymerization, the mass experiences slight thermal expansion, so the pressure inside the mold does not rise as sharply as during hot polymerization. At room temperature, polymerization of most materials occurs in 20-30 minutes. Acceleration of curing can be achieved by immersing the mold in water heated to 37 °C. When preparing the molding compound, it is necessary to take into account that volumetric shrinkage depends on the monomer/polymer ratio and increases with increasing this ratio.
Linear shrinkage (taking into account technological methods) of self-hardening plastics averages from 0.15 to 0.5%.
The powder/liquid ratio recommended by the manufacturer must be strictly followed.
The rate of polymerization of self-hardening plastics depends on the following factors:
- 1) initial temperature of the monomer and polymer; high temperature (above 30 °C) causes rapid polymerization; upon cooling (below 5°C), the process slows down sharply, and at negative temperatures the reaction practically stops;
- 2) the amount and nature of the activator and initiator;
- 3) the degree of dispersion of the powder and its molecular weight: the finer the powder and the lower the molecular weight, the faster the swelling and polymerization occurs;
- 4) monomer/powder ratio. Reducing the monomer/powder ratio shortens the polymerization time.
An excess of monomer slows down the process, but at the same time a higher temperature of the polymer is observed and shrinkage increases, which ends after 3 hours. The polymerization process, as already noted, is exothermic. The heat of polymerization of the MMA monomer is 78.7 kJ/mol.
When mixing powder with liquid, the resulting molding mass retains its plasticity and the temperature does not increase noticeably. The induction period at point A (Fig. 21) turns into a rapid process of development of the polymerization reaction, and the temperature quickly rises. After curing is complete, the temperature of the polymer decreases due to heat transfer to the environment. The temperature jump and the duration of the induction period, which determines the viability of the compound, depend on the mass of the polymerizing polymer-monomer mixture, the redox system and the initial temperature of the liquid and powder. With an increase in mass to 50 g, a sharp increase in the temperature jump is observed. The dependence of the thermal effect on the size of the polymerizing mass results in a higher monomer conversion in the thick parts of the product (prosthesis, etc.). This means that thin sections of the product have relatively lower mechanical strength because they contain more residual monomer. Due to the fact that the temperature during polymerization of self-hardening plastics is below 100 ° C (the boiling point of the monomer is 100.3 ° C), the polymers are distinguished by the absence of pores and cavities caused by boiling of the monomer. Depending on the type of work, the molding compound is used at various stages of swelling.
- Stage I - sand. It appears immediately after mixing the powder with the liquid and, depending on the ambient temperature, can last from 30 s to 5 minutes or more. At a temperature of 10 °C it lasts about 5 minutes, at 15-18 °C - 3 minutes, at 18-22 °C - 1-2 minutes, and at 25 °C it ends in 0.5-1 min. In the sand stage, the monomer-polymer mixture is unusable.
- Stage II - viscous, stretchy threads. The initial period of this stage is characterized by the appearance of stretchy threads, stickiness of the mass, high plasticity and fluidity. At the beginning of the second stage of swelling, the molding mass is used for work requiring adhesion. The molding compound applied to the prosthesis base forms a strong connection after curing.
- Stage III - dough-like. The molding compound in this swelling stage is characterized by loss of stickiness, good ductility and less fluidity. In this state, the molding mass can be conveniently formed on plaster models, producing protective palatal plates, replacing, shaping and obturating dentures, Porta splints, individual trays, orthodontic devices and other dental structures. The mass can be used for relining dentures in all cases, as well as when it is necessary to obtain an imprint of the relief of the prosthetic bed in conditions of functioning dentures, when significant chewing pressure can develop.
- Stage IV - rubbery. At this stage, the molding mass retains its given shape even with minor short-term mechanical impact on it. When relining, the denture is removed from the oral cavity when the molding mass is already in the rubber-like stage. In the case of relining partial dentures with the presence of converged and diverged teeth in the oral cavity or teeth with well-defined equators, the dentures are removed from the oral cavity only after reaching a rubber-like state. Removal at stage III of swelling will result in distortion due to tension. If you skip stage IV, the plastic will harden and the prosthesis cannot be removed from the mouth without sawing. When monitoring the curing of the polymerizing mass, it is necessary to pay attention to the thinner areas of the prosthesis, since they cure more slowly than the thick ones. It should be noted that the polymerization of the monomer-polymer system from the beginning of mixing to curing is a continuous process without sharp interstage transitions.
Optimal pressing mode for products made from self-hardening plastics . The main method of processing self-hardening plastics, which ensures the production of a high-quality product, is pressing. For self-hardening plastics, an important processing parameter is determining the moment of application of pressure. If pressure is applied before the required time, the product is obtained with large shrinkage and unsatisfactory surface quality. Products with the required accuracy can be obtained only with a sharp increase in specific pressure. The working time of self-hardening plastics is significantly affected by changes in ambient temperature even by 2-3°C, and this circumstance causes difficulties in determining the moment of application of pressure (see Fig. 21). The methods used for manufacturing dental structures from self-hardening compounds at room temperature without pressure are not optimal. The polymer is less dense and has lower physical and mechanical properties.
One of the possible options for optimizing the technology of pressing products from self-hardening plastics is to carry out the final stage of polymerization under compressed air pressure. In Fig. Figure 22 shows an apparatus for polymerizing products made of self-hardening plastics. It is a hermetic vessel, inside of which a pressure of 0.3-0.5 MN/m2 is created by air heated to 40-45°C. Inside the apparatus there are shelves on which products are placed for polymerization. Control and maintenance of the set temperature is carried out using a thermocouple interlocked with a temperature relay and an electric heater. The device can be made by converting the ultrathermostat UT-15.
A rack is placed in a preheated apparatus, on which plaster models with products made from self-hardening molding mass, which is in a rubber-like stage, are installed. The device is sealed and a pressure of 0.3-0.5 MN (3-5 atm) is created. The pressure is controlled using a pressure gauge. If the pressure exceeds, the safety valve is activated. After 15-20 minutes, the finished products are removed from the apparatus.
Comparative characteristics of hot and cold curing plastics . Self-hardening plastics are inferior to hot-curing plastics in a number of indicators, but this is compensated by their exceptional ease of use and better dimensional stability. Polymerization of self-hardening plastics is accompanied by lower monomer conversion, so they contain 5-10 times more residual monomer. This leads to faster aging of the polymer and a decrease in strength characteristics. As a result of leaching of the monomer from the surface of the product, the structure of the polymer is loosened, which leads to a change in a number of properties of the product. Thus, when the monomer content in the polymer decreases from 8.5 to 0.9%, the heat resistance increases from 52 to 130°C, and the Brinell hardness increases from 70 to 194 MN/m2. Self-hardening plastics (linear) exhibit higher hygroscopicity (water absorption>0.7 mg/cm2) than hot-curing plastics and contain large amounts of residual benzoyl peroxide, monomer, activator, which is a prerequisite for the deterioration of their physical and mechanical properties over time. Research has shown that the main factor distorting the size and shape of the prosthesis is not polymerization shrinkage, which is compensated by technological methods, but thermal shrinkage that occurs when the prosthesis is cooled from the polymerization temperature to room temperature. Since the polymerization of self-hardening plastics occurs at lower temperatures than hot-curing plastics, dentures and other dental products made from self-hardening plastics are more accurate and are better fixed in the oral cavity. In addition, less stress occurs in them, although they are inferior in strength to hot-curing plastics, but they are more flexible. Their modulus of elasticity is 2•20-3 MN/m2, and for hot-curing plastics it is 3.8•103 MN/m2 (Table 12). With additional heating and holding for several hours, the physical and mechanical properties of products made from self-hardening plastics can be somewhat improved by reducing the content of residual monomer. The domestic industry produces self-hardening base material protacryl-M.
Features of application
It is necessary to use products for medical purposes only if mixed in the correct proportions.
Features of the use of materials:
- The liquid is mixed with the powdered composition until a viscous substance is formed. Excess monomer can lead to increased material shrinkage, porosity and poor coloration.
The mixed composition is tightly closed. Over a half-hour period of time, the mass swells and goes through several stages: sandy, viscous, dough-like, rubbery. - The polymerization mode occurs when immersed in water or heated and the presence of an activator. The individual regimen must correspond to the description on the packaging.
It is important to follow the instructions exactly, because if the polymerization process is shortened, there will be an excess of monomer, which will provoke inflammatory processes in the mucous membrane. If overheated, it will become excessively brittle. - During the production of prostheses, the formation of porosity in the material is possible. This phenomenon occurs during accelerated polymerization and when there is a lack of monomer.
If the proportions are observed, the listed phenomena and deformation can be avoided. Depending on the stage of swelling, specialists use the material for filling, making devices, and making impressions.
The video discusses the properties of quick-hardening plastic for the manufacture of prostheses.
Method for making a temporary splint from quick-hardening plastic
Several methods have been proposed for the manufacture of temporary splints from quick-hardening plastics.
We present the method described by M.R. Marey. To make a splint using this method, it is necessary to make an aluminum mold in the form of a groove, concave in the shape of the dental arch. The mold is made from an aluminum plate 1 cm wide, 0.25-0.30 mm thick and 13-14 cm long (Fig. 168). In the absence of a plate, it can be obtained by flaring ordinary aluminum wire 2-3 mm thick. In order to obtain the shape of the groove, the plate is stamped on a lead tile with wire 3-3.5 mm thick (Fig. 168). Before bending the mold along the dental arch, it is annealed on the flame of an alcohol burner. This makes it soft and pliable. Its edges are bent with crampon tongs so that they do not bend inward, but, on the contrary, diverge outward. The latter circumstance allows you to subsequently remove the mold from the hardened tire without much difficulty. Applying a splint consists of the following technical techniques:
1. Applying a ligature to the teeth to be splinted. As a ligature, take a plastic thread (fishing line) 0.3-0.4 mm thick, which is tied with a triple knot on the vestibular surface of the tooth (Fig. 168). The ends of the thread are cut short.
2. Preparation of dough from quick-hardening plastic.
3. Formation of the tire. The prepared dough of quick-hardening plastic is placed in a mold pre-greased with Vaseline and pressed against the teeth so that the ligature nodes are immersed in the plastic. Excess plastic that extends beyond the edges of the mold is removed with a spatula. After the plastic has hardened, the aluminum mold can be easily separated from the tire with a spatula. Irregularities and sharp protrusions on the tire are removed with carborundum heads. The splint should not lie on the gum, and applied to the lower row of teeth should not interfere with the closure of the teeth. The splint ligatures should not be inserted into the gum pocket.
Source
Reviews
Plastics have the ability to quickly harden under normal conditions, so they are widely used in medical practice for filling, manufacturing and repairing devices.
You can share your feedback on the use of plastics in the comments to this article.
If you find an error, please select a piece of text and press Ctrl+Enter.
Tags prosthetics removable dentures
Did you like the article? stay tuned
Previous article
Types of complications that arise after dental prosthetics and treatment tactics
Next article
What justifies the popularity of French TBR implants in many countries around the world?
New trends in the production of base polymers
In the last few years, there has been a tendency to introduce aesthetic fibers into the structure of hot-curing base polymers. They look more advantageous, but they are inferior to the standard ones in terms of physical and mechanical properties. “Veins” in the structure of polymers lead to the formation of microvoids and reduce the density of the material. Oxygen in microvoids increases the number of monomers. Therefore, such polymers with improved aesthetic characteristics should be used only when indicated - in rare cases.
Despite certain disadvantages, acrylic plastics remain the most common material for the production of removable denture bases. Their main advantages are low price, manufacturability and lack of need for expensive equipment.