Ketorol
NSAIDs have a pronounced analgesic effect, have anti-inflammatory and moderate antipyretic effects. The mechanism of action is associated with non-selective inhibition of COX activity (COX-1 and COX-2), which catalyzes the formation of prostaglandins from arachidonic acid, which play an important role in the pathogenesis of pain, inflammation and fever. Ketorolac is a racemic mixture of [-]S- and [+]R-enantiomers, with the analgesic effect due to the [-]S-form. The strength of the analgesic effect is comparable to morphine, significantly superior to other NSAIDs.
The drug does not affect opioid receptors, does not depress respiration, does not cause drug dependence, and does not have a sedative or anxiolytic effect.
After oral administration, the analgesic effect develops within 1 hour.
Pharmacokinetics
Suction
When taken orally, ketorolac is well and quickly absorbed from the gastrointestinal tract. The bioavailability of ketorolac is 80-100%, Cmax after oral administration at a dose of 10 mg is 0.82-1.46 mcg/ml, Tmax is 10-78 minutes. Food rich in fat reduces the Cmax of the drug in the blood and delays its achievement by an hour.
Distribution
Plasma protein binding is 99%, Vd is 0.15-0.33 l/kg. The time to reach Css when taken orally at a dose of 10 mg 4 times a day is 24 hours, Css is 0.39-0.79 mcg/ml.
Excreted in breast milk: when taking ketorolac at a dose of 10 mg, Cmax in breast milk is achieved 2 hours after taking the first dose and is 7.3 ng/ml, 2 hours after taking the second dose of ketorolac (when using the drug 4 times a day) – 7.9 ng/l.
Metabolism
More than 50% of the administered dose is metabolized in the liver with the formation of pharmacologically inactive metabolites. The main metabolites are glucuronides and p-hydroxyketorolac.
Removal
Excreted mainly by the kidneys - 91%, through the intestines - 6%, glucuronides are excreted in the urine. Not excreted by hemodialysis.
T1/2 in patients with normal renal function averages 5.3 hours (2.4-9 hours after oral administration at a dose of 10 mg). When administered orally at a dose of 10 mg, the total clearance is 0.025 l/h/kg.
Pharmacokinetics in special groups of patients
T1/2 increases in elderly patients and shortens in young ones.
Impaired liver function does not affect T1/2.
In patients with renal failure, the Vd of the drug may increase by 2 times, and the Vd of its R-enantiomer by 20%. In patients with impaired renal function with a plasma creatinine concentration of 19-50 mg/l (168-442 µmol/l), T1/2 is 10.3-10.8 hours, with severe renal failure - more than 13.6 hours. In patients with renal failure (with plasma creatinine concentrations are 19-50 mg/l) total clearance is 0.016 l/h/kg.
Instructions for use KETOROL gel 2%
- The gel should not be applied to the eyes, mucous membranes, or areas of skin with open lesions, dermatoses or infectious lesions. Do not touch sensitive areas of the skin during the procedure and until your hands are cleansed of drug residues.
In some patients, the drug may cause allergic reactions. If irritation or inflammation occurs at the application site, stop using the drug.
In patients with a history of allergic reactions, ketorolac can cause the development of severe anaphylactic reactions; there have been cases of complications of this kind in patients who have not previously had allergic reactions. There is a possibility of cross-hypersensitivity with acetylsalicylic acid, phenylacetylic acid and other NSAIDs.
Ketorolac gel should be used with caution in surgical patients with a known bleeding tendency or who are receiving other drugs that prolong bleeding time. All topical NSAIDs may slow wound healing.
Information on excipients
:
The medicine contains dimethyl sulfoxide and propylene glycol, which may cause skin irritation.
The drug contains sodium methyl and propyl hydroxybenzoate, which can cause allergic reactions, including delayed ones.
Impact on the ability to drive vehicles
:
With systemic use of ketorolac, cases of drowsiness and decreased ability to concentrate have been reported, however, with local use, the likelihood of developing such a complication is extremely low due to the insignificant systemic absorption of the drug.
Use for liver dysfunction
:
Ketorolac should be used with caution in patients with impaired liver function or a history of liver disease. When using ketorolac, an increase in liver enzyme levels may occur. If signs of liver dysfunction are detected during the use of ketorolac, treatment should be discontinued.
Use in patients with impaired renal function
:
Ketorolac should be used with caution in patients with impaired renal function or a history of kidney disease.
Laboratory indicators
:
After topical application of Ketorol gel, the concentration of ketorolac in the blood serum is very low, however, it is known that ketorolac, like other inhibitors of prostaglandin synthesis, can cause an increase in the level of urea and creatinine in the blood, inhibition of platelet aggregation and, as a result, an increase in blood clotting time. Therefore, it is recommended to stop using the drug 48 hours before determining liver function tests, urea and creatinine levels, and blood clotting time.
Ketorol gel (2% 30gl)
INSTRUCTIONS for the use of the medicinal product for medical use KETOROL ®
Registration number: LP-001080
Trade name of the drug: Ketorol®
International nonproprietary name of the drug: ketorolac.
Dosage form: gel for external use.
Composition Each 1 g of gel contains: Active substance: ketorolac tromethamine (ketorolac trometamol) – 20 mg; Excipients: propylene glycol 300 mg, dimethyl sulfoxide 150 mg, carbomer 974P 20 mg, sodium methyl parahydroxybenzoate 1.8 mg, sodium propyl parahydroxybenzoate 0.2 mg, tromethamine (trometamol) 15 mg, purified water 390 mg, flavoring "Drimon Inde" (triethyl citrate 0 .09%, castor bean seed oil 0.14%, isopropyl myristate 0.30%, diethyl phthalate 24.15%) 3 mg, ethanol 50 mg, glycerol 50 mg.
Description Homogeneous transparent or translucent gel with a characteristic odor.
Pharmacotherapeutic group: non-steroidal anti-inflammatory drug (NSAID)
ATX code: M01AV15
Pharmacological properties Pharmacodynamics Nonsteroidal anti-inflammatory drug (NSAID) has a pronounced analgesic and anti-inflammatory effect. The mechanism of action is associated with non-selective inhibition of the activity of cyclooxygenase (COX) - COX-1 and COX-2, which catalyzes the formation of prostaglandins from arachidonic acid, which play an important role in the pathogenesis of pain, inflammation and fever. Ketorolac is a racemic mixture of [-]S and [+]R enantiomers, and the analgesic effect is due to the [-]S form.
When applied topically, it causes a weakening or disappearance of pain at the site of application of the gel, including pain in the joints at rest and during movement, reduces morning stiffness and swelling of the joints. Helps increase range of motion.
Indications for use
Local application for pain relief:
- for injuries (soft tissue bruise, soft tissue inflammation, including post-traumatic origin, ligament damage, bursitis, tendinitis, epicondylitis, synovitis);
- for pain in muscles (myalgia) and joints (arthralgia), neuralgia, radiculitis, rheumatic diseases.
Intended for symptomatic therapy, reducing the intensity of pain and inflammation at the time of use, does not affect the progression of the disease.
Contraindications
- hypersensitivity to ketorolac or other components of the drug;
- weeping dermatoses, eczema, infected or open wounds (at the site of application of the gel);
- complete or incomplete combination of bronchial asthma, recurrent nasal polyposis or paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (history of bronchospasm, urticaria or rhinitis caused by taking acetylsalicylic acid);
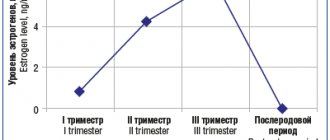
- pregnancy (III trimester);
- period of breastfeeding (lactation);
- children up to 16 years of age.
Precautions for use Exacerbation of hepatic porphyria, erosive and ulcerative lesions of the gastrointestinal tract, severe renal/liver failure, chronic heart failure, bronchial asthma, old age, pregnancy (I and II trimester).
Directions for use and doses
For external use only.
Before applying the gel, wash and dry the skin surface. Apply a uniform thin layer of gel about 1-2 cm long to the area of maximum pain 3-4 times a day. The application of the drug is carried out with soft massaging movements, through which the gel is distributed over the skin over the affected area.
The drug should be reused no earlier than after 4 hours. Use the drug no more than 4 times a day. Do not exceed the indicated dose.
If symptoms persist or worsen, or there is no improvement after 10 days of using the drug, you should stop treatment and consult a doctor. Do not use the gel for more than 10 days without consulting a doctor.
Possible side effects when using the drug
Local reactions: itching, urticaria, peeling.
If any adverse reactions occur, you should stop using the drug and consult your doctor.
When applying the gel to large areas of the skin, the development of systemic adverse reactions cannot be ruled out: heartburn, nausea, vomiting, diarrhea, gastralgia, ulceration of the gastrointestinal mucosa, increased activity of “liver” transaminases; headache, dizziness; fluid retention, hematuria; allergic reactions (anaphylactic shock, skin rash); thrombocytopenia, leukopenia, anemia, agranulocytosis, prolongation of bleeding time.
Symptoms of overdose, measures to assist in case of overdose Cases of overdose with gel have not been described. If you accidentally ingest the gel, you must empty your stomach (by inducing vomiting, taking activated charcoal) and consult a doctor. Further treatment, if necessary, is symptomatic.
Interaction with other drugs Pharmacokinetic interaction with drugs that compete for association with blood plasma proteins is not excluded. Caution should be exercised when using ketorolac simultaneously with digoxin, phenytoin, lithium preparations, diuretics, cyclosporine, methotrexate, other NSAIDs, antihypertensive and antidiabetic agents. Before using the gel, you should consult your doctor if you are using these products or are under medical supervision.
special instructions
It is recommended to apply the drug only to intact areas of the skin, avoiding contact with open wounds. Avoid getting the gel into the eyes and other mucous membranes. Do not use the gel under airtight dressings. After applying the gel, wash your hands with soap. Close the tube tightly after using the gel.
Release form
Gel for external use 2%. 30 g in a laminated aluminum tube, equipped with a membrane to control the first opening. Tube with instructions for use in a cardboard box.
Storage conditions
At a temperature not higher than 25 °C. Do not freeze! Keep out of the reach of children!
Best before date
2 years. Do not use after the expiration date stated on the packaging.
Conditions for dispensing from pharmacies
Over the counter.
Manufacturer Dr. Reddy's Laboratories Ltd., India Dr. Reddy's Laboratories Ltd., India
Manufacturing Address : Khol, Nalagarh Road, Baddi, Dist. Solan, Himachal Pradesh, India.
Information about complaints and adverse drug reactions should be sent to the following address: Representative office: 115035, Moscow, Ovchinnikovskaya embankment, 20, building 1 tel. fax