Author of the article:
Soldatova Lyudmila Nikolaevna
Candidate of Medical Sciences, Professor of the Department of Clinical Dentistry of the St. Petersburg Medical and Social Institute, Chief Physician of the Alfa-Dent Dental Clinic, St. Petersburg
For many years, every second person in the street was convinced that a good toothpaste must certainly contain fluoride. Our grandparents stubbornly insisted on the importance of this element. But is fluoride really necessary in toothpaste, or does the harm of this microelement still outweigh its benefits? Let's try to figure it out.
The history of fluoride in toothpastes
Fluoride was first used in toothpastes more than 100 years ago, in 1914 in the USA. At the beginning of the 20th century, scientists found that this element is an excellent protection against caries and its use reduces the rate of tooth decay by an average of 30-45%. Under the influence of fluorine, enamel became resistant to acids and more durable.
Why is fluoride needed in toothpaste?
Surprisingly, the same element can be both healing and destructive to the body. And fluoride is just that: the substance can both restore and strengthen tooth enamel, and cause irreparable harm to it. It is thanks to fluoride that the strength of bone tissue in the body is maintained; this element plays a key role for the health of hair, nails and teeth. Fluoride is very important in the growth and development of a child: without it, his skeleton does not develop normally.
In addition, fluorine has a beneficial effect on metabolism: without this element, the body cannot remove heavy metals. The substance also helps absorb iron and supports immunity.
When there is a lack of fluoride in the body, bones and teeth are the first to suffer. Bones bend, become brittle and brittle, and in the event of a fracture they heal very slowly. With a lack of fluoride, the enamel gradually becomes thinner, it is increasingly affected by plaque bacteria, and caries develops faster and faster.
In fact, it is not difficult to provide the body with its daily requirement of fluoride. Products high in this substance include:
- a variety of fruits, such as grapefruits and apples;
- nuts;
- dairy products;
- various types of meat (especially liver);
- various vegetables - pumpkin, spinach, potatoes;
- buckwheat and oatmeal porridge;
- honey;
- any types of tea.
By drinking enough water per day and eating right, you can provide yourself with fluoride. Therefore, the need for toothpastes with fluoride should only be determined by a dentist. As a rule, fluoride products are prescribed to smokers and people who abuse coffee.
Fluorine and fluorides
Fluorine in its pure form is a yellowish gas with a characteristic pungent odor. It is poisonous, so it should be used with extreme caution in domestic conditions. Among all the elements of the periodic table, it is one of the most chemically active. Compounds of fluorine with other substances are called fluorides: there are a huge number of them, so their areas of application are also very extensive. This includes dentistry. The first pastes containing fluoride appeared at the beginning of the twentieth century, and in the late 1940s, an experimental program for water fluoridation began in the United States, which brought mainly positive results in the fight against caries. After some time, several dental associations of the leading countries of the world recognized fluoridated toothpastes as an effective preventive measure. At the same time, the number of opponents of fluoride increased, including quite well-known specialists.
Attention!
Toothpastes and other oral care products use fluoride rather than fluoride itself.
Pros of fluoride toothpastes
Many people specifically choose hygiene products with fluoride to strengthen their teeth and prevent the development of caries. Indeed, in some cases, this element protects the enamel from the action of harmful bacteria and strengthens it. Under the influence of fluoride, less acid is released, which affects the enamel. Therefore, anti-caries toothpastes often contain fluoride.
The main benefits of fluoride toothpastes include:
- antiseptic effect;
- improvement of metabolism;
- improvement of the remineralizing effect of saliva;
- stimulation of the salivary gland;
- inhibition of the transformation of soft plaque into tartar.
Is fluoride harmful or beneficial?
So, fluoride is an actively used component in most toothpastes. This substance reduces the likelihood of developing caries and strengthens the enamel. Complete refusal from at least periodic use of fluoride-containing toothpastes increases the rate of caries development by about a third and contributes to the development of tartar and plaque.
The most effective compounds containing fluoride are amino fluoride and sodium fluoride, therefore, when choosing a paste in the store, you should carefully look at what exactly is included in its composition.
During cleaning, fluoride, unless, of course, the paste is intentionally swallowed, does not enter the bloodstream.
Fluoride-containing pastes should not be used:
- young children, as they often swallow the paste;
- residents of regions where the amount of fluoride in water significantly exceeds the norm (for example, this is observed in Odintsovo, Krasnogorsk, Tver, Serdobsk, Yegoryevsk and other settlements).
For other categories of people, fluoride does not pose any threat.
Sometimes you can hear opinions that fluoride is not just harmful, but even dangerous. The only harm that can be caused by excess fluoride is provoking the development of fluorosis, and even then the disease affects exclusively children whose teeth have not yet begun to erupt. There are no proven cases of the development of fluorosis from the use of pastes: the disease is detected only in children living in regions with the amount of fluoride in drinking water above 1 mg per liter.
You can find many articles online, the authors of which claim that fluoride is extremely dangerous. However, there is no evidence of this.
Harm of toothpastes with fluoride
Unfortunately, many of us forget that toothpaste is not the only source of fluoride. This element is found in many foods and even in ordinary tap water.
Before you buy even the best toothpaste with fluoride, it is important to understand your need for this element: its daily intake should not exceed three milligrams. In order to get this dose, it is enough to drink two liters of water a day.
For a person who eats right and drinks enough water, it is not at all necessary, and often even harmful, to use toothpastes with fluoride. The fact is that this element in excess amounts is toxic to the body. The substance accumulates in tissues, including tooth enamel. If too much fluoride accumulates in it, fluorosis appears, the process of destruction of enamel.
Fluorosis is very easy to recognize. The disease manifests itself as white spots on the surfaces of the teeth. These spots grow quickly, turn yellow and begin to corrode the enamel. In case of fluorosis, it is important to start treatment as soon as possible - remineralizing therapy, photophoresis or the use of special applications.
Often, teeth with fluorosis require additional whitening, because it is almost impossible to cope with such a problem with the help of professional cleaning.
How do toothpastes work to restore enamel?
Anyone who cares about dental health has probably asked the question more than once: “how can you strengthen tooth enamel?”
If your teeth have become sensitive or stains have appeared on your teeth, this is certainly a reason to consult a doctor, sort out the problems and choose treatment; home care may not be enough; serious in-office remedies may be needed.
However, as a preventative measure, strengthening the enamel is possible with the help of properly selected toothpastes. To understand, we will first delve a little deeper into chemistry.
Let's understand the processes in tooth enamel, pay attention to the composition of toothpastes, and by combining this knowledge, you will be able to choose your own toothpaste to restore teeth, but do not forget to consult with your doctor.
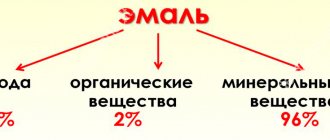
So, tooth enamel is the hardest tissue, which consists of 96% of inorganic mineral compounds - phosphorus, fluorine, calcium and other components. But despite her hardness, she is quite vulnerable. To find out why, let's delve a little deeper into chemistry.
The main compound of enamel is hydroxyapatite, and few people know that in the surface layer of enamel, hydroxyapatite crystals undergo constant changes. When the pH of saliva becomes acidic (in other words, teeth are not brushed), the hydroxyl group from our hydroxyapatite turns into water!
The crystal lattice is broken, calcium is released, and the vulnerability of the enamel to acids has increased. This is the process of demineralization of enamel at the molecular level. If there is an excess of calcium or phosphorus ions in the periodontal environment, then the reverse process will begin - mineralization.
What about fluoride? And fluorine ions form a modification - hydroxyfluorapatite is a fairly acid-resistant compound, and in high concentrations calcium fluoride is a generally insoluble compound.
In conditions of healthy oral microflora and normal saliva composition, the natural processes of demineralization and remineralization occur constantly and ensure a balanced mineral balance of tooth enamel.
The source of calcium and phosphate ions is saliva, and poor hygiene and poor nutrition contribute to the proliferation of bacteria, which in turn contribute to the accumulation of acids in the oral cavity.
So daily brushing of teeth is not just a hygienic procedure, it is a war for the strength and durability of the enamel of your teeth.
Indications for strengthening enamel
- The main indications for strengthening tooth enamel are:
- Caries at any stage.
- Baby teeth.
- Pregnancy and breastfeeding period.
- Discomfort or toothache while eating.
- Time before and after teeth whitening procedure.
- Orthodontic treatment (braces).
- Chips, scratches and cracks on teeth.
- Bite abnormalities and, as a result, pathological abrasion.
- Long-term drug therapy.
How do toothpastes work to restore enamel?
Most researchers agree that the main source of substances entering the enamel is oral fluid. There is also a very important detail: enamel has high permeability, due to the presence of microspaces filled with water through which various substances can penetrate, depending on their radius and activity. For example, the fluoride ion is large and active, therefore it is embedded quickly and only into the surface layers of the enamel, while the calcium ion is the opposite, so it manages to penetrate much deeper.
All this was proven through long-term research and based on these details, remineralization schemes were created - artificial saturation of tooth enamel with minerals.
Since ions penetrate the enamel through a slow diffusion process, remineralization requires time and therefore multiple manipulations.
Most of all, tooth enamel needs an element such as calcium. The strength and hardness of teeth depends on it. Calcium-containing pastes are suitable for restoring the enamel layer with minor damage. They contain various calcium compounds that penetrate dental tissue, strengthen and restore them.
Toothpastes with calcium are especially useful for primary signs of demineralization. Damaged enamel, which may periodically show itself with increased sensitivity, needs to be healed with pastes containing active calcium;
In case of serious damage to the enamel layer, it is recommended to use fluoride-containing pastes. Pastes containing fluoride, when cleaning the oral cavity, actively react with calcium ions, filling microcracks and damage to the enamel.
Penetrating into the enamel layer, the substances are integrated into the structure of the tissue, restoring and strengthening it.
It is useful to alternate fluoride-containing paste and calcium paste. It is undesirable for both components to be present in one tube, since together they form an insoluble salt. Because of this, when brushing your teeth, active fluoride and calcium ions will not be released from the paste, so there will be no benefit from such therapeutic cleaning. Modern research does not stand still, constantly modifying and improving the compositions of toothpastes. But there are still “old” toothpastes on the market that do not provide any benefit to teeth. I recommend carefully studying the composition of toothpastes and consulting with your dentist; some of them are reliable and proven
Toothpastes for restoring enamel with calcium
We have already seen that calcium ion has very weak activity. Therefore, it is very important that its form in toothpaste is easily absorbed by tooth enamel.
Calcium in toothpastes can be contained in the form of the following compounds:
- Calcium citrate.
- Calcium pantothenate.
- Calcium lactate.
- Hydroxyapatite.
Very often you can find a combination of two or three of these calcium compounds in toothpaste, which, of course, increases the chances of calcium being incorporated into the tooth enamel, and therefore strengthening the teeth.
Calcium citrate replenishes calcium deficiency in the body and is safe to swallow. The calcium in this compound easily penetrates tooth enamel, strengthening and protecting it.
Calcium pantothenate is a widespread calcium compound present in the body and actively involved in metabolism. It enriches teeth with essential calcium and is safe when ingested.
Calcium lactate is used in the food industry to fortify foods with calcium and as a substitute for table salt. It is perfectly absorbed by the body and protects against caries.
“Good old” calcium glycerophosphate. It has been established that toothpastes based on calcium glycerophosphate effectively remove plaque, stabilize the cariogenic situation in the oral cavity and do not have a sensitizing effect on the body. Pastes containing calcium glycerophosphate are time-tested, affordable and reliable. For all conservatives for complex therapy of superficial caries.
For those who love innovation.
Nanotechnology has reached toothpastes. Hydroxyapatite itself is by no means an innovation in dentistry. Experiments on its use took place at the beginning of the 20th century, but only at the end did Japanese scientists manage to develop nanocrystalline medical hydroxyapatite. This artificially synthesized form is maximally biocompatible and bioactive.
Over time, the formula has been improved, the particles have decreased, and today toothpastes containing nanohydroxyapatite are leading in the prevention of caries. Its effectiveness has been proven in the initial stages of caries, due to the ability of ions to penetrate into the microscopic spaces between enamel prisms, embedding themselves in the crystal lattice of hydroxyapatite crystals of tooth enamel. As a result, tooth enamel is restored.
Toothpastes with nanohydroxyapatite eliminate caries at the white spot stage and “seal” microcracks; reduce tooth sensitivity.
An indispensable assistant at the first signs of problems with tooth enamel.
Another contender for special treatment
Amorphous calcium phosphate. When in contact with saliva and hydroxyapatite, this element forms a special biofilm on the surface of the teeth, which: protects the enamel from the harmful effects of acids and ensures the connection of bioavailable calcium with the enamel, accelerating its remineralization.
Important! Since the active substance is obtained from cow's milk casein, this method of strengthening enamel is not suitable for people with allergies to milk protein.
Theobromine is for lovers of natural and maximally safe, expensive and chic. Theobromine (cocoa bean extract) acts as a catalyst; it stimulates the formation of its own hydroxyapatite crystals, providing natural remineralization of enamel. Calcium acetate serves as a building material for the crystal lattice of tooth enamel.
The enamel becomes denser and more resistant to acids. Studies have confirmed the beneficial effects of theobromine on tooth enamel and its high effectiveness in combating tooth sensitivity.
Please note that forms of calcium not listed above are merely abrasives in toothpaste (for example, calcium carbonate or dicalcium phosphate). Therefore, when choosing a toothpaste to strengthen your teeth enamel, be guided by the calcium compounds described above, or consult your dentist.
Toothpastes for strengthening enamel with fluoride
Every person knows: fluorides protect teeth from caries! But there is other known information - fluoride is toxic to the body. How to be? Who to believe? Let's figure it out.
In our body, elements such as fluorine and calcium are divided into macroelements, there should be a lot of them, and microelements, they are needed, but in small quantities. For example, the daily calcium intake for an adult is 1000-1200 mg. While the norm for fluoride is only 4 mg. But, did you know that with an excess of calcium and vitamin D in the body, the risk of urolithiasis increases. And excess iron in the blood also causes toxic effects and is called iron overload syndrome. And so the list can be endless.
Important! Everything useful is useful in moderation! Same with fluorine.
How to understand whether it is needed and whether it will not be harmful?
First of all, determine what fluoride content is in the water in the region where you live. Since we get the most fluoride from drinking water. You can find up-to-date information about fluoride levels in water in your area on the website of your local water utility.
In Russia, the fluorine content in water from natural sources is usually low - less than 0.5 mg/l. Only in the groundwater of the Tver, Moscow, Ryazan, Sverdlovsk and Chelyabinsk regions the concentration of fluorine is increased (reaches 4.4 mg/l). There is also a list of regions where, due to large production, the fluoride content is also increased.
Only in regions with high levels of fluoride in water are toothpastes with fluoride contraindicated. Everyone else needs and benefits from fluoride in the right dosages.
What are the benefits of fluoride?
Fluoride performs important tasks in the body.
- Together with phosphorus and calcium, it participates in the formation and strengthening of bone tissue and tooth enamel.
- Promotes healthy nail and hair growth.
- Stimulates hematopoietic processes: formation, development and maturation of red blood cells, platelets and leukocytes.
- Strengthens the immune system and maintains it at an appropriate level.
- Removes salts of radionuclides and heavy metals from the body.
- When used in oral care products, fluoride helps prevent dental problems and solve existing ones.
- Prevent tooth decay. Fluoride penetrates the enamel structure, prevents caries and treats it at the white spot stage.
- Protect against demineralization. The combination of fluorine with hydroxyapatite forms fluorohydroxyapatite, a substance that reduces it. This is how enamel remineralization occurs.
- Protect against lactic acid. Fluoride prevents bacteria from producing lactic acid. This reduces the growth of pathogenic microflora and gives the enamel additional protection.
Why is fluoride deficiency dangerous in the body?
Fluorine deficiency in the body is most often caused by its low content in drinking water (less than 0.7 mg/l). Another possible cause is improper regulation of fluoride metabolism in the body. A deficiency of this substance weakens tooth enamel, making it more vulnerable to caries. A child with a lack of fluoride may experience delayed ossification and defects in bone mineralization. In an adult, with prolonged deficiency, the risk of developing osteoporosis increases.
If there is too much fluoride in the body
Fluoride is found not only in oral care products and tap water, but also in food. The maximum permissible daily intake for an adult is 10 mg. An excess of fluoride is more dangerous for the body than a deficiency, as it entails irreversible processes. First of all, teeth and bones are affected; metabolic disorders, deterioration of blood clotting, etc. may occur. In children, even before teething, endemic fluorosis develops - this is a chronic lesion of tooth enamel in the form of spots of various sizes, shapes and colors. After 10–20 years of excess fluoride in the body, bone fluorosis develops, which can develop into osteosclerosis, osteoporosis and osteosarcoma (malignant formation). As a rule, we are talking about excess fluoride entering the body for a long time with water and food or, for example, toxic production. It is also possible that fluoride metabolism in the body is impaired, which is rare.
Is fluoride from toothpaste harmful?
We figured out that the toxic effect of fluoride, which some fear, is due only to ingestion. Should you be afraid of fluoride toothpaste? No! When brushing our teeth, we do not swallow toothpaste. And fluorine, as we remember, an element with high activity, quickly reacts with hydroxyapatite of tooth enamel, settling in its surface layers and does not have time to penetrate through the tooth into the bloodstream. This means that when brushing your teeth 2 times a day, toothpaste with fluoride does not cause any harm. And the concentrations of fluoride in toothpastes are strictly limited. The amount of fluoride in ppm is usually indicated on the packaging. The abbreviation ppm stands for parts per million and reflects the number of fluorine particles per million. If the tube states that the toothpaste contains 900 ppm of fluoride, this means that 1 kg(!) of toothpaste will contain 900 mg of this element.
The higher the fluoride concentration, the better the paste restores enamel. For prophylactic pastes it is 900-1150 ppm, for therapeutic and prophylactic pastes it is 1350–1500 ppm. There are products with higher content, but they are used only as prescribed by a doctor and in short courses. As a reminder, these recommendations are suitable for anyone living in areas with moderate levels of fluoride in their drinking water.
Can children have fluoride toothpaste?
The European Academy of Pediatric Dentistry (EAPD) recommends fluoride pastes and gels for everyone - even children and pregnant women - to prevent early stages of tooth decay.
Recommended fluoride content in toothpaste:
- up to 4 years - 200 ppm;
- from 4 to 8 years - 500 ppm;
- over 8 years old and for adults - 1450 ppm.
Fluoride is an important component in caring for children's teeth. However, if the toothpaste is swallowed frequently, fluoride accumulates in the body and can cause unpleasant consequences. Important! The child brushes his teeth under the supervision of an adult!
Children can begin brushing their teeth with fluoride-containing toothpastes when their first tooth erupts. Because the recommended amount of toothpaste when brushing your teeth is a grain of rice. And the amount of fluorine is 200-250ppm. That is, it is 200 mg in 1000 mg paste. Tube with children's toothpaste 50ml. Even if we roughly translate, without taking into account the density of the substance, that ml is equal to mg, then we get 10 mg of fluorine. It turns out that for the toxic effect of fluoride to manifest itself, the child must eat the entire tube of toothpaste at one time! Agree, if a child eats a whole tube of cream or anything else, this will also cause problems. Therefore, it is recommended to keep any attractive tubes away from children. And the child brushes his teeth under adult supervision.
How do fluorides affect enamel?
Fluoride ions interact with calcium ions, forming calcium fluoride, which is built into the structure of tooth enamel, restoring and strengthening it. Therefore, in order to fix calcium in the teeth and slow down the carious process, it is recommended to use fluoride-containing products to restore teeth. After all, as we discussed at the beginning of the article, hydroxyfluorapatite is a more acid-resistant compound, and calcium fluoride is insoluble, which means that enamel with such structural elements is stronger and less susceptible to caries. In addition, fluorine has a bacteriostatic effect, which helps to heal the microflora. This is why fluoride toothpastes are so popular among dentists.
However, all fluorine-containing additives have their own characteristics and differ in the speed of action. For example, amino fluoride, which is an organic fluorine compound, forms a persistent protective film on the surface of the enamel. Inorganic fluorine compounds (sodium fluoride and others) also form a protective film, but under the influence of saliva it dissolves faster.
As in the story with calcium, we pay attention to the composition.
Sodium monofluorophosphate. Today it is rare, in old-style toothpastes. It excludes all fluoride compounds. It does not contain fluorine ion, but a solid compound that does not dissolve as easily as ionic compounds. This compound was the result of creative work by chemists as a solution to the problem of combining calcium and fluorine.
What happens to this compound in the oral cavity remains unclear even to the scientists themselves who studied this topic. Some claimed that the element immediately binds to tooth enamel, others said that upon contact with enzymes in saliva, fluoride ion is released. But the secret remained not fully revealed!
Sodium fluoride is the most common supplier of fluoride. It is the simplest fluoride compound: fluorine ion and sodium ion, which are tightly bound to each other due to their charge. Thus, sodium fluoride is a salt that dissolves very easily in water, as well as in saliva (although not as well as regular table salt). Sodium fluoride supplies tooth enamel with fluoride ions, and thereby overcomes the first step in the prevention of caries.
Aluminum fluorides
Also a salt, as in the case of sodium fluoride, but has limited solubility in water, similar to calcium fluoride. Thus, since fluoride ions have a cariesstatic effect, the prospects for protection when using aluminum fluoride compounds are very limited.
For this reason, preference for choosing a product based on it remains minimal.
Zinc fluorides - today only in combination with other fluoride compounds.
Zinc fluoride is also a salt, easily soluble in water. In principle, a good candidate for joining the ranks of toothpaste formulations. Indeed, the first toothpaste with fluoride was based on zinc fluoride, a well-known American brand. But highly reactive zinc ions tend to cause problems! Therefore, the creators are forced to resort to some secrets to stabilize it. This has caused most manufacturers to switch to the more stable sodium fluoride.
In recent years, zinc fluorides have been experiencing a renaissance! Since in combination with amino fluorides they form a stable and effective chain.
Aminofluorides are the product of targeted development and long research!
Amino fluorides are also salts, but compared to sodium fluorides they have a more complex structure. They were the result of a focused search for the best compound to protect teeth from caries. The fluorine ion in the case of amino fluorides is bound into a complex amine molecule by fatty acid hydrolase. The peculiarity of these compounds is their bipolarity: on the one hand, the polar amine base is soluble in water, on the other hand, the nonpolar base is fatty. Such compounds become active on the surface, enveloping it.
| Component | Name on the tube | A comment |
| Sodium monofluorophosphate | Sodium monofluorophosphate | It is ineffective because due to the rate of decay it begins to act only after 3–4 minutes of cleaning. |
| Sodium fluoride | Sodium fluoride | Has a powerful antibacterial and restorative effect. The active substance reduces the ability of bacteria to convert sugar into acid, which destroys enamel. |
| Aminofluoride | Aminofluoride/Olaflur | Recognized as the most effective for caries prevention. Forms a protective film on the surface of the teeth, from which fluoride enters the enamel and strengthens it. |
| Tin fluoride | Stannous fluoride | It is proven effective, but has a side effect: it first lightens the restored areas of enamel, and then leads to their noticeable darkening. |
For home care and caries prevention, you can use toothpastes with fluoride; the most effective are those containing sodium fluoride and amino fluorides. The higher the fluoride content, the better the remineralizing properties of the paste and the protection against caries. But it is better not to use high dosages without a doctor’s prescription.
Fluoride and calcium are equally important for enamel remineralization. However, we remember that when combined they form an insoluble salt and neutralize each other’s effects. Now some manufacturers are trying to get around this problem. In the meantime, follow the proven scheme: alternate the use of pastes with calcium and fluoride.
Which toothpaste is best for strengthening enamel?
Of course, the one that suits you. When making a choice, you should not listen to commercials or the advice of friends.
The state of dental health varies from person to person. Advice from a dentist after a routine examination of the oral cavity will certainly help you decide on the right choice of toothpaste with active ingredients for the restoration and prevention of tooth enamel.
Proper nutrition, regular oral hygiene procedures and the use of only high-quality and highly effective toothpastes will help strengthen the enamel and maintain the health of your teeth.
Indications for the use of fluoride pastes
Of course, in some cases, toothpastes with fluoride will be useful to patients, but, as we have already said, only a dentist should prescribe them. The specialist will assess the condition of the enamel and oral cavity, and then determine whether the teeth have enough fluoride and what kind of toothpaste they need.
Therapeutic toothpastes with calcium and fluoride are indicated for use if the patient has:
- signs of dental demineralization;
- poor enamel resistance to acids;
- fragility of teeth, tendency to chip;
- minor signs of caries.
The fluorides contained in such toothpastes create a thin protective layer on the crowns. All substances hazardous to enamel do not penetrate it and their negative impact is reduced several times. Incoming fluoride, when it is deficient in the body, slows down the proliferation of bacteria and, accordingly, the development of caries. The likelihood of gum inflammation is also reduced.
How does fluoride affect teeth?
Whether fluoride is beneficial or harmful to teeth - there is no definite answer. The human body needs this substance only in a reasonable amount, otherwise it can cause the formation of various diseases.
Fluoride performs the following functions in the body:
- It is a preventative against oral diseases, such as caries and periodontal disease.
- Takes part in the process of formation of tooth enamel.
- Actively cleans hard dental tissues.
- Has antibacterial properties.
- Has a positive effect on the soft tissues of the oral cavity.
Consequences of excess fluoride in the body
Do not forget that fluorine is, first of all, a toxic substance. It is added to toothpastes only in the form of compounds that can release active ions. The pastes contain sodium fluoride, sodium monophosphate, as well as tin and aluminum fluorides, and amino fluoride.
When fluoride enters the body of an adult in excess quantities, the following problems may begin:
- disruption of metabolic processes and, as a result, destruction of bones and teeth;
- disruptions in the functioning of the endocrine system;
- liver and kidney diseases;
- increased blood pressure;
- decreased immunity.
Surprisingly, translated from Greek, the word fluorine means “destroying.” Fluorine compounds in the gaseous state are deadly. And fluorites, often added to toothpastes, are considered one of the most harmful substances for the body.
If you use fluoride paste constantly, without apparent need, the following changes may occur in the oral cavity:
- damage to the enamel, the appearance of stains on it;
- fragility of teeth;
- increased sensitivity of teeth.
Treatment
Treatment goals: avoid the harmful effects of fluoride and eliminate existing negative symptoms. It is recommended to drink plenty of milk or calcium-rich drinks. Calcium reacts with fluoride to form poorly soluble calcium fluoride, which is not easily absorbed.
Additional recommendations and treatment regimen depending on age and fluoride dosage.
Age
|
Children's toothpastes with fluoride
Children's toothpastes should absolutely not contain fluoride, despite the fact that this element is necessary for growing organisms. Fluoride is added only to toothpastes for children and adolescents over 5 years of age. The thing is that young organisms perceive various components much more strongly and are many times more susceptible to fluorosis (excess fluoride).
So, now you know the benefits and harms of fluoride in toothpastes. You should not decide on the use of such means on your own. Only an experienced dentist should prescribe pastes with fluoride and calcium.
To avoid the need for medicinal pastes, take good care of your teeth and be attentive to your body. Do not get carried away with coloring products, coffee, tea and sweets. For dental health, the absence of bad habits, proper, balanced nutrition and daily thorough hygiene procedures are important.
An excellent tooth support product is ASEPTA “Remineralization” professional toothpaste with high concentrations of hydroxyapatite and thermal mud. This remedy restores tooth enamel, reduces the sensitivity of teeth and gums and accelerates the regeneration of the oral mucosa.
What are the dangers of fluoride deficiency and excess?
Low fluoride content weakens tooth enamel, making it more susceptible to tooth decay. But excess fluoride is more dangerous than its deficiency. The teeth and bones are the first to suffer - they become fragile. Metabolic disorders, blood clotting and other serious health problems can also be observed.
So, at the moment, science has not proven the harm of fluoride-containing toothpastes, but there are many facts indicating their benefits. Which toothpaste should you choose for quality dental care? Your dentist will recommend the best option based on the current clinical situation, your complaints and wishes.
Clinical researches
Clinical studies have proven that regular use of professional toothpaste ASEPTA REMINERALIZATION improved the condition of the enamel by 64% and reduced tooth sensitivity by 66% after just 4 weeks.
Sources:
- Report on the determination/confirmation of the preventive properties of personal oral hygiene products “ASEPTA PLUS” Remineralization doctor-researcher A.A. Leontyev, head Department of Preventive Dentistry, Doctor of Medical Sciences, Professor S.B. Ulitovsky First St. Petersburg State Medical University named after. acad. I.P. Pavlova, Department of Preventive Dentistry
- Study of the clinical effectiveness of treatment and prophylactic agents of the Asepta line in the treatment of inflammatory periodontal diseases (A.I. Grudyanov, I.Yu. Aleksandrovskaya, V.Yu. Korzunina) A.I. GRUDYANOV, Doctor of Medical Sciences, Prof., Head of Department I.Yu. ALEXANDROVSKAYA, Ph.D. V.Yu. KORZUNINA, asp. Department of Periodontology, Central Research Institute of Dentistry and Maxillofacial Surgery, Rosmedtekhnologii, Moscow
- Clinical studies of antisensitive toothpaste “Asepta Sensitive” (A.A. Leontyev, O.V. Kalinina, S.B. Ulitovsky) A.A. LEONTIEV, dentist O.V. KALININA, dentist S.B. ULITOVSKY, Doctor of Medical Sciences, Prof. Department of Therapeutic Dentistry, St. Petersburg State Medical University named after. acad. I.P. Pavlova