Description of the substance
What is borax substance? Many minerals containing sodium tetraborate have been studied. Such deposits include:
- borax or sodium tetraborate tinkal decahydrate;
- kerite;
- Borax sediment is formed during the drying up of salt lakes (Searles, lakes of Turkey);
- minerals belonging to the borate class, containing calcium, sodium and similar elements other than borax.
Varieties of borax
There are two options for this material. In solid form, borax appears as a powder with small granules of a small fraction with a white color. The flux will not spread during application and can be precisely positioned in the required metal-to-metal junction areas.
Diluted borax has also been used for light metals. In some cases, liquids are very convenient, because you just need to dip a small part into the solution. In addition, the liquid form allows the use of borax even at low heating temperatures.
In some cases, it is advantageous to use a mixture of fluxes with borax included in the composition for the special characteristics of the base metal and special requirements for the connection.
Indications for use
Before purchasing a solution, you must consult a specialist. The drug is intended for local use and is effective for the following pathologies:
- Fungal infection of the vagina;
- Bedsores;
- Diaper rash;
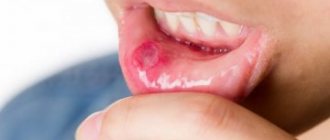
- Stomatitis;
- Pharyngitis;
- Tanzillite.
Treatment and prevention of fungal infections with sodium tetraborate in glycerin should be discussed with your doctor. Therapy for vaginal candidiasis should be carried out by medical personnel, since if the procedures are carried out independently, there is a risk of relapse.
Composition and properties
The chemical nomenclature of soldering borax indicates that it is a crystalline hydrate of the sodium salt of tetraboric acid. If the substance contains 10 water molecules, then it is called sodium tetraborate decahydrate. In simple words, it is a salt that is surrounded by a shell containing 10 or 5 water molecules.
A temperature of 64 degrees causes decahydrates to melt and lose water in the process. Borax is dehydrated at a temperature of 380 degrees Celsius. Tetraborate is characterized by withstand heating up to a temperature of 742 degrees and melting when it increases.
Borax contains sodium chlorine, barium chlorine and, in some cases, boric acid. Flux in the form of a solution has a high ability to dissolve metal oxides, as well as fatty films and anything unnecessary that can prevent the adhesion of materials.
Thanks to the use of borax during soldering, many products are produced without defects.
Designation
The name BORAX is usually reserved for the name "disodium tetraborate decahydrate". However, the terms "borax anhydrous" and "borax pentahydrate" are also found in the technical and scientific literature for other forms of disodium tetraborates most widely used in industry:
- Disodium tetraborate (synonyms: boric acid , disodium salt, anhydrous borax, anhydrous borax).
- Disodium tetraborate decahydrate (synonyms: borax, borax decahydrate, tetraboric acid salt, sodium borate, boric salt, E285, borax, sodium borate, boric acid, sodium tetraborate).
- Disodium tetraborate pentahydrate (synonyms: borax pentahydrate, borax hemihydrate).
Kinds
Based on appearance, welding drills are divided into 2 types.
- Solid. In powder form, flux has the form of solid fine fractions. This shape makes it easy to lay borax on a metal surface before the soldering process, while the substance does not spread. Solid borax is sold in boxes that are sealed, thereby protecting the substance from moisture and the negative influence of the environment. In the powder fraction, borax is white.
- Divorced. This type of borax is considered the most suitable for light metal and its alloy. The substance is the same powdered borax, but dissolved in a liquid. This feature of the flux makes it possible to use it at low soldering temperatures. Using diluted borax is quite simple: small metal elements are dipped into it and then soldered. This flux is popular in jewelry, as well as when working with wires and contacts.
Instructions for use
To use sodium tetraborate for medical purposes, you must carefully study the instructions that come with each dosage form and follow the advice of your doctor. When using glycerin-based borax, doctors usually advise diluting it with water or chamomile infusion.
When making slimes, a borax solution (activator) is always added at the last stage, when all other components have already been mixed. Add it gradually, 1 drop at a time, stirring thoroughly each time. To thicken the slime, only a few drops of solution are required, but an overdose can lead to structural damage, loss of elasticity, and this process is irreversible.
Advantages and disadvantages of the product
Let's start with the pros:
- The drug is extremely affordable. The cost of a bottle of borax does not exceed $1, which is several times cheaper than other antiseptics and combination products.
- The drug is non-toxic, well tolerated and does not cause serious side effects.
- The product can be used in problem categories of patients: children, pregnant women, nursing mothers.
- The drug is effective, especially in gynecology. Glycerin has a softening and anti-inflammatory effect, relieving itching and burning, and boric acid helps restore the acidic protective environment of the vagina, stimulating the growth and reproduction of beneficial lactic acid bacteria.
Directions for use and dosage
Depends on the diagnosis. The solution should be used by rinsing, lubricating, douching twice a day. It is worth deciding in advance where to order the drug; you can also use the delivery service.
When treating stomatitis in children and adults, on the recommendation of a pediatrician and therapist, use a cotton or gauze swab soaked in a solution. Treatment of the oral cavity should be carried out 2 times a day for 6 days. If the symptoms of a fungal infection have resolved earlier, treatment must be continued to avoid relapse.
Before treating thrush, you need to perform preparatory procedures. After pre-treatment, a cotton or gauze swab is moistened in the solution and inserted into the female genital organs for 30-35 minutes. During this time, the patient should be in a supine position with the pelvis elevated.
For mild symptoms of candidiasis, the procedure is carried out once a day. In the chronic form of the disease, 2 times a day. The number of procedures in the treatment of thrush is determined by the doctor, based on the patient’s condition and the results of therapy.
Treatment of the upper respiratory tract is carried out by lubricating the tonsils. To do this, you can use a cotton or gauze swab. The procedure must be repeated 5-6 times a day until the symptoms of the disease completely disappear.
One bottle of the drug is enough for one period of treatment. It turns out to be quite economical, since the price at the pharmacy does not exceed 100 rubles. For such a price, every buyer can afford the drug.
Scope of application of the drill
Sodium tetraborate has been actively used for the following purposes:
- as a flux during soldering and melting of metals;
- in analytical chemistry studies as a standard substance for determining the level of acid in a solution. Borax is also used to establish the characteristics of metal oxides;
- widespread use in the creation of glazes, enamel, glasses for optical instruments and decoration;
- the powder is used in pharmaceuticals and paper production;
- is a natural preservative and means for disinfection and control of parasites;
- is a component in the chemical industry to create household cleaning products;
- relevant application in the creation of cosmetic products;
- borax is used as a base for creating boron;
- the substance is a component for creating insulating building materials;
- in light industry, sodium tetraborate is applied to the product before the painting procedure.
Using a drill in everyday life
Borax can be found in most grocery stores. It is a relatively inexpensive product, making it an excellent choice for many household projects.
Use of sodium tetraborate as a medicine
The substance is very effective in controlling pests: cockroaches, ants and other household insects. The mixture is ready by mixing equal parts of powder with sugar. Sugar helps attract beetles and borax exerts its detrimental effect on the insect. It is recommended to keep the substance in hard-to-reach places, away from pets and children. Optimal places: under stoves, refrigerator and sink. Borax also works well against mice. You just have to apply the powder in the areas where the mice are located, and the borax will rid you of the pests. You can also sprinkle the solution on the carpet and vacuum it to eliminate the presence of fleas or treat the mattress to get rid of bedbugs.
Borax will get rid of rust. Mixing 1 cup of powder with 2 cups of water and 1 tablespoon of lemon juice makes an effective anti-corrosion agent. The paste-like solution is applied to the rusty items for about 15 minutes. After which the rust can be easily removed by mechanical friction.
Sodium tetraborate is a universal cleaner. Two tablespoons of borax are mixed with 2 cups of water to create an all-purpose cleaner. The solution can be applied in a spray bottle and used to clean kitchen surfaces and bathroom tiles and ceramics. Borax is great for removing very stubborn stains. Borax will help remove stubborn stains from the floor.
Borax will allow you to flush your home plumbing fixtures. Simply place ½ cup of borax into the drain with a few cups of warm water. Borax breaks down dirt that gets stuck in pipes. This will not only remove excess and unclog the drain, but also disinfect the system.
Application in forging and forge welding
Borax is actively used as a flux for forging and forge welding. The powder is a source of boron oxide, with excellent antioxidant properties. Borax can, if necessary, remove small cracks during metal processing, change the shape of a product, or when heated during artistic forging and blacksmithing of metal. The substance is classified as a high temperature flux. The processed workpiece with borax is characterized by more wear-resistant characteristics and durability.
Using borax in soldering.
During the melting of borax at a temperature of 700-900 °C, the surface of the material being processed is cleaned, and all excess inclusions are dissolved in the flux. During the processing of a material by forging, a thick layer of scale is gradually created. In some cases, the metal being processed may completely burn due to overheating of the part. But by using a thin layer of borax, this scenario can be avoided.
In industry
Industrial enterprises find the following uses for this substance:
- as a raw material for the production of boric acid;
- as an antiseptic in the production of bulk insulation materials such as “Ecowool”.
- as a means for determining the concentration of acid solutions;
- in welding production as a component of flux;
- in the mechanical engineering industry as a component of antifreeze and various lubricants;
- in glass production;
- the fabric is treated with sodium tetraborate for better dyeing;
- Borax is a component of almost all cosmetic products.
Using borax in the soldering process
In addition to household use, borax is a popular raw material for soldering. High temperature flux is produced as a finely dispersed powder. When heated above 700 degrees Celsius, the flux flows into a liquid state and becomes solder. I’ll tell you about the pros and cons of this flux in the table below.
| Pros of borax flux | Cons of solder |
| The elements being connected do not need to be heated to the same temperature. | After the borax solder cools, salt-type deposits form on the surface of the seam, which will have to be cleaned off from time to time. This is not very convenient for hard-to-reach places. |
| The quality of the roller is high even when connecting different materials. For example, metal and non-metal. | |
| The roller can be destroyed if necessary by reheating the borax to the melting point. | |
| The peak heating temperature of 700+ C will not allow copper parts to warp + the characteristics of the weld with this solder are higher than usual. | Flux absorbs moisture very well. A closed storage container doesn't even help. Too wet solder reduces the quality of the roller several times. |
| Capillary soldering itself is considered the best solution for connecting small and medium-sized parts. | |
| The powder mixture makes it possible to achieve a high level of seam strength. |
To learn how to use Borax without jambs, you need to maintain clear proportions when applying. For beginners in welding, this can be problematic, so gaining practical experience with such solder is extremely important.
Rules for soldering with technical borax:
- The base for applying boron powder must first be cleaned. The welder gets rid of various greasy stains, wax and other debris from the surface of the future seam. For this procedure I use regular sandpaper. It's quite enough.
- The soldering iron tip needs to be preheated before touching the solder.
- To form a reference seam, I advise you to heat the workpieces to the same temperature.
- The tip of the soldering iron should heat both sides of the elements being connected.
- Do not skimp on solder to form a quality connection.
- When the solder begins to flow, the soldering iron tip is temporarily removed from the joint location.
- After applying the bead, the joined elements cannot be moved until they have completely cooled. When working with transistor components, you should use “crocodiles” that will act as heat sinks, thereby reducing the risk of temperature damage to the filling.
If dry joints occur, the roller must be melted and the seam redone. After finishing work with solder, the tip of the soldering iron is cleaned. When working with copper parts, cleaning the edges of the elements being joined is especially important.
What tools and materials are used in the borax soldering process:
- soldering iron Used to heat solder + sometimes for the connections themselves. Sometimes it is more convenient to heat the elements with a gas burner;
- stand. To position the soldering iron while it is heating up or is inactive. There are a lot of variations of stands for this instrument on the Internet, so choosing one that is convenient for you will not be any problem;
- rag or sponge. Consumables needed to clean the soldering iron tip;
- fine grit sandpaper. Used for stripping joints to which solder will be applied;
- crocodiles. The clamps play an auxiliary role as radiators;
- burner. Needed when soldering pipes.
Also, to improve the quality of the seam, I advise you to use boron flux, which will contain fluoride or chloride elements. When soldering pipes, the elements being connected must be heated for at least 25 seconds.
How to make borax with your own hands?
Features of the soldering process
There are various soldering options. Many of them have a similar operating algorithm.
Submerged Soldering
For a quality connection, the following steps are necessary:
- The solder must be applied to a clean surface that acts as a base. It is necessary to remove any oils, paints, waxes and other impurities using a solvent, steel brush, or fine sandpaper.
- In order for the solder to connect to the tip of the soldering iron, heat it up for a few seconds, and only then apply the solder. Hold the soldering iron like a handle, near the base of the tool.
- Both parts of the workpiece that will be soldered must be hot to form a good bond.
- The tip of the soldering iron heats both sides of the workpieces.
- The solder will not flow well on the heated base metal. Enough solder should be used to form a strong connection.
- Remove the tip from the joint area as soon as the solder begins to flow.
- Do not move the solder joint while the solder is cooling. Do not overheat the connection as this may cause damage. Transistors and some other components may be damaged due to the heat of soldering. The alligator clip can be used as a heat sink to protect these components. By absorbing heat, the alligator clip reduces heat, helping prevent damage.
- Soldering a connection can only take a few seconds, and even an amateur can perform the operation. If the seam looks bad, reheat it and try again. Bad bonds (also called dry bonds) must be melted and remade. Wipe the tip of the soldering iron to clean it. Unplug the soldering iron when not in use.
Read also: Calculation of cutting power during turning
What is it and what is it for?
Soldering borax is a high-temperature type of powdered flux that is used when joining metal products by soldering. Melting of this substance can occur under the influence of temperatures exceeding 700 degrees Celsius. Soldering borax has its own GOST, according to which it is manufactured and its characteristics are regulated.
The substance in powder form looks very similar to salt, in other words it is called sodium tetraborate. The synthesis of borax occurs naturally, and its extraction is carried out from salt lake deposits.
The use of this substance is quite wide, but most often it is used for soldering copper pipes.
The advantages of using borax include the following:
- the materials that are planned to be processed may have different temperature conditions;
- obtaining a high-quality, reliable weld not only between metals, but also between metal and non-metal surfaces;
- ease of soldering seams if necessary to separate parts;
- when soldering, the parts do not warp or deform;
- increased productivity during capillary soldering;
- obtaining smooth and durable seams even from a craftsman with little experience.
The disadvantages of sodium tetraborate are as follows:
- release of a large volume of salts, which harden on the metal at high speed;
- absorption of moisture from the environment;
- the difficulty of selecting the right amount of borax for an inexperienced welder.
Description and external signs
Borax mineral is an aqueous sodium borate with hydroxyl. It is also popularly known as boric salt. In Asian countries it is called tincal. This substance resembles almost colorless crystals. Origin of the name: from the Arabic word bauraq - translated as “white”. The form of release of borax in pharmacies and hardware stores is a white fine-crystalline powder.
Chemical composition
The components of borax are the sodium salt of boric acid and a strong base (water, sodium). Chemical formula: Na2(B4O5)(OH)4 8H2O
The practical significance of borax is to serve for the extraction of boron.
Molecular weight of the substance: 381.37; Density: 1.7; Specific gravity: 1.69 – 1.8; Maximum birefringence: δ=0.025; IMA classes: borates.
physical characteristics
The color of borax can vary: it can be white with a gray tint, yellowish, less often with a blue or greenish tint. When exposed to light, the crystals are almost colorless. The gloss is matte resinous. The stone looks as if it has been oiled. In terms of hardness, it is a very brittle mineral. Borax is highly soluble in water and forms a slightly alkaline, sweetish solution. Melting point – 60°C; at 320°C the crystals lose water and a white powder is formed.
How to replace sodium tetraborate for slime?
There are no complete analogues of Borax. But there are substances that are similar in action.
You can get rid of mold using copper sulfate or soda. Soda will help in the fight against cockroaches.
Furacilin is suitable as an antiseptic for the treatment of stomatitis and the upper respiratory tract.
But replacing sodium tetraborate to thicken slimes is much easier.
- Substitutes can be found at a hardware store (soda ash, Persil laundry detergent, Fairy dishwashing detergent, Air wick air freshener).
- At the pharmacy (alcohol solution of boric acid, Teymurov foot spray, Vizin eye drops, lens cleaner).
- And even in the kitchen you can make an activator from baking soda, table salt, gelatin, and starch.
Of course, these thickeners are not as reliable as Borax, and more quantities are required, but they can also replace the usual activator if necessary.
Flaws
- After use, a deposit of salts forms, which must be removed mechanically;
- It is necessary to choose storage areas that are free of moisture, since high humidity will cause the flux to deteriorate;
- To prepare the material for use, you need to spend time and choose the right proportion, which can lead to errors.
Varieties of Borax
There are two main varieties that relate to the appearance of the material. The first option is the solid form. Borax soldering flux is supplied in the form of a powder with fine solid fractions. Thanks to this, it is easy to lay it on the metal surface before soldering in the required quantity and it will not spread at the same time. This variety is supplied in a special box that protects the material hermetically from the penetration of moisture and other foreign factors. Fractions are white.
Soldering borax in powder form
The second type, which is more often used for lighter metals and their alloys, is diluted borax. In this case, you are offered the same material, but dissolved in liquid. Due to this, it can be used at lower soldering temperatures. Using this type is also easier, since small parts are simply dipped into the liquid, after which they can be soldered. This is used both in the jewelry industry and in other places where small items are worked. Contacts, wires and other types of equipment come into good contact with dissolved flux. Despite the fact that the principle of using borax for soldering in liquid form is somewhat different from the standard one, they have almost the same effect.
There are also varieties in the form of mixtures, when other fluxes are also used. This is necessary in cases where it is impossible to achieve the desired results using one substance. Proportions and composition depend on specific goals. Most often it is combined with boric acid.
Composition and physicochemical properties
The composition of borax for soldering includes sodium chloride and barium chloride, in some cases boric acid is added to it. It is not used in its pure form for all procedures, since this would require too high a melting point. Drill soldering powder is a high-temperature flux, so its main property is resistance to high temperatures. It is worth noting that the material perfectly retains its chemical properties even at a lower concentration than what is supplied. Thus, the flux solution has a fairly high level of dissolution of the oxides of all base metals for which it is used.
It can also dissolve fatty films and other unnecessary things that will interfere with the normal soldering of the material. Brown soldering protects against many types of defects that can occur in work.
Specifications
There are two main grades of the substance, which are defined according to GOST as grade A and grade B:
Soldering of metals is carried out by first removing traces of oxides from their surface. Fluxes are used for this. They should prevent oxidation when heated and encourage good flow of molten solder.
For soldering copper products, borax solder ideally meets all requirements. The substance has been known since the Middle Ages. It was mined in the lakes of India and Tibet, then transported to Europe, where it was used for processing fabrics and leather, and producing glass.
Borax is widely used for working with metals. When manufacturing or repairing metal products, borax soldering is carried out. First of all, the method is used for parts made of copper and brass. A special type of this flux is used when repairing jewelry.
Applications of sodium borate
- In thermochemical processes in the production of metals;
- Glass industry: insulating glass fibers or glass wool, glass fibers for the textile industry, fibers for plastic reinforcement.
- Ceramic industry, in the production of porcelain enamels;
- In cosmetology for skin cleansing;
- As a preservative for sturgeon caviar;
- In taxidermy;
- In the production of various insecticides;
- When cleaning metals to dissolve rust;
- Production of perborates and other boron .
- In the manufacture of safety glasses resistant to high temperatures;
- In the manufacture of glasses;
- In the production of detergents, disinfectants, soaps and pesticides;
- In analytical chemistry;
- As a fertilizer for plant nutrition;
- In the making of hand forged tools such as knives and swords;
- As part of flux in the manufacture of gold products.
Side effects
A side effect to the drug can only appear if there is a high sensitivity to the components.
These include:
- Itching
- Weakness
- Possible skin rashes
- Convulsions
- Digestive disorders
- Dermatitis
- Failure of the menstrual cycle
- Abdominal pain
- Cardiopalmus
- Anemia
You should stop using this drug if you experience any side effects and seek immediate medical attention.
Contraindications
- Mechanical damage to the skin and mucous membrane
- Breastfeeding and pregnancy
- Hypersensitivity to individual components of the drug component
- Drug intolerance
Authenticity
Qualitative reaction. To 5 ml of a 4% solution add 0.1 ml of a 0.1% phenolphthalein solution; the solution turns red. When adding 5 ml of glycerin, the 85% solution should become colorless.
- Qualitative reaction. To 0.2 g of the substance add 1 ml of concentrated sulfuric acid, 3 ml of 96% alcohol and mix. When ignited, the mixture should burn with a green-edged flame.
- Qualitative reaction. A 4% solution should give a characteristic reaction A to sodium (General Pharmacopoeia Monograph “General reactions to authenticity”).
Disadvantages of flux
When working with borax, a characteristic deposit remains on the surface of the base metal, which must be mechanically cleaned off. Borax is susceptible to moisture and should be stored in a dry place. It is necessary to carefully prepare the flux in advance so as not to spoil the product.
Types of soldering flux
Tools and materials
The soldering technology uses a number of components.
- A soldering iron is used to heat joints that are to be soldered. Solder has a lower melting point than the metals that are being joined. Solder melts when heated with a soldering iron.
- Borax acts as a flux to prevent oxidation of the metals that are combined.
- The solder used to join copper pipes has a necessary acid base that is suitable for pipes but is corrosive to electronic connections.
- A stand on which you can hold a hot soldering iron. There are various stands. It is important to always keep the hot soldering iron in place when not in use.
- A sponge or rag that is used to clean the tip of an iron.
- Fine sandpaper used to clean connections before soldering.
- Alligator clips can be used as heat sinks if necessary.
- Burner if pipes are soldered.
Tools for soldering
Thus, the use of borax is effective in domestic conditions for cleaning surfaces and parts, and also as an antiseptic. The ingredient is often used for soldering various parts as protection against oxidation and corrosion prevention. Low cost and widespread use have allowed the substance to be used in many areas of industry and installation services.
Type of substance
Borax, or borax as it is also called, is a preservative. Preservatives are used in the food industry to increase the shelf life of a product. All chemical compounds belonging to this type of substance have one thing in common - they have powerful antibacterial properties. It is due to this that products to which preservatives are added do not spoil for a long time.
Despite its beneficial properties, the preservative E285 is very toxic and dangerous for the human body. Therefore, in Russia it is not used in the food industry.
Toxicological properties and characteristics
Warm-blooded animals and humans. Sodium tetraborate penetrates the skin and has a mild cumulative effect. Does not have a local irritant effect upon contact with the conjunctiva of the eye and skin, does not cause a sensitization effect or an embryotoxic effect. [2]
People who work with the substance often suffer from chronic eczema. When working, it is necessary to protect the respiratory system, eyes and skin from exposure to dust. [3]
Sodium tetraborate, as well as boric acid and soluble borates, are quickly and almost completely absorbed from the gastrointestinal tract. In the blood, boron is evenly distributed between red blood cells and plasma, but quickly passes into the tissues. Found in soft tissues
10% of the dose (mainly in the brain, liver and adipose tissue). The excretion of boron compounds occurs mainly through the gastrointestinal tract. [3]
Hazard classes. Insecticides based on sodium tetraborate belong to class IV of low-hazard disinfestation agents according to GOST 12.1.007. [2]
Properties
| Index | Standard values |
| Color | white |
| Compound | sodium tetraborate |
| Appearance | crystalline substance |
| Smell | absent |
| Solubility | dissolves in cold water (1:25), boiling water (2:1), glycerin; does not dissolve in alcohol |
| Density | 1,73 |
| Other | antiseptic |
The main thing about borax with glycerin
In appearance, borax with glycerin has a transparent 5–20% solution with a slight specific odor.
Sodium tetraborate contains:
- the main substance is borax or sodium tetraborate decahydrate (20 g)
- excipient - glycerol or glycerol (80 g).
Borax with glycerin is available in glass bottles and with a nylon and tightly screwed plastic cap, 30 g each. Borax itself acts as an antiseptic, or rather eliminates various infections, and glycerin removes irritation and helps the borax penetrate skin barriers better. Using glycerin, the solution becomes thick.
Is borax sold to children in pharmacies?
Sodium tetraborate is sold in pharmacies in the form of an aqueous or glycerin solution. The antiseptic belongs to the group of low-toxic drugs and is available without a prescription. Therefore, if desired, even a schoolchild can purchase a bottle with a slime activator. By the way, you won’t be able to buy it in a pharmacy in Kazakhstan.
In 70% of cases, the danger lies in the toxicity not of sodium tetraborate itself, but of the compounds that are obtained in the manufacture of slimes. It is not advisable to mix borax with phenols:
- methanol;
- resorcinol;
- pyrogallol.
Chemical reactions occur when sodium tetraborate is mixed with strong acids, which are part of household chemicals.
Application of brass and copper powder
Practitioners often use flux that has been stored longer than expected. To solder with brass, the borax must be re-melted. The cooled powder should be placed in a jar with an airtight lid. Neglecting this procedure can ruin the work due to waste accumulated during storage.
At the beginning of soldering, the working area must be heated to a clearly visible red color. Heating should begin first at the edges, and then directly at the soldering site.
Then the heated area should be gradually sprinkled with flux, wait until it spreads in the form of a film along the edges of the part. At this point, the heated brass solder must be dipped into the molten borax so that it is covered with a hot flux film.
As experience shows, the soldering area is red in color, while the borax melt is colored bluish. You cannot keep solder in the flux for a very long time. Oxide residues may form.
Then you should warm up the work area again. The brass will take on an orange glowing appearance. You can proceed directly to soldering. If done correctly, the solder will fill all the gaps.
The soldering area will turn golden. When the process is completed, the hot zone should be sprinkled with borax powder and left to cool. Copper parts in a hot (200 ℃) state can be placed in a mixture containing equal parts acetone and water, or simply in water. It makes sense to immerse the cutters in hot sand.
A correctly made connection has a transparent film with a slight blue tint. There are no solder drops on it. If soldering is performed incorrectly, the seam becomes covered with a black porous crust.
The reason may be overheating of the working area, as a result of which slag is formed, or poor quality of borax-based flux. This is how brass and other copper-containing alloys are soldered.
Copper pipe connection
Copper pipelines are expensive. The investment can be justified with careful installation, which is often carried out by capillary soldering using borax as a flux.
It is worth noting that today, other fluxes are sold that are more convenient to use. One pipe is inserted into the second or fitting so that the gap does not exceed 0.4 mm.
Soldering time is short, 3 minutes. It is important that the parts remain stationary during operation. In order for the borax powder to stick to the surface, the copper is first heated with a torch.
For pipes with a diameter of up to 108 mm, the soldering process is carried out at low temperatures not exceeding 450°. The seam is wide (up to 50 mm), but not very strong. Wide pipes with a diameter greater than 159 mm are soldered at high temperatures. Only professionals can perform the procedure.
In both cases, the solder melt penetrates well into the capillaries of the parts, which contributes to the formation of strong connections. It is recommended to remove any remaining borax.
It must be remembered that soldering is accompanied by the formation of smoke, so you can only work in ventilated areas.
How to make borax at home?
The cost of sodium tetraborate in hardware stores and pharmacies varies from 20 to 100 rubles. If we are talking about a one-time use, then the price is cheap, but if a person constantly needs flux or borax for other tasks, it would be more rational to get it at home. In the table below I will describe 3 popular recipes for making the substance with your own hands.
| № | Components | Packing (g) | Preparation |
| 1. | Baking soda | 50 | Pour boric acid into a glass container and add baking soda in small portions. Stir until completely dissolved. Next, we do filtration - we pass the mixture through special paper or a napkin. You should get about 100 grams of sodium tetraborate. |
| Boric acid | 70 | ||
| 2. | Water | 200 ml | Finely chop the laundry soap using a grater. To start, 1 teaspoon is enough. Pour 200 ml of water into a glass container, add boric acid and gradually add crushed laundry soap. Place the mixture in the microwave for a couple of minutes. We filter and get borax. |
| Boric acid | 5 | ||
| Laundry soap | 50 | ||
| 3. | Powdered borax | 20 | This recipe involves obtaining sodium tetraborate in liquid form. Simply mix borax powder with glycerin and leave for a day. |
| Glycerol | 80 |
Although borax itself is not a highly toxic substance, constant exposure to human skin can lead to dermatitis. If a person, due to his profession, periodically interacts with this inorganic compound, I strongly recommend using gloves, safety glasses and a respirator.
What kind of workwear should a welder have?
This concludes the analysis of the concept of what borax is. I hope that the information provided was of value to you. Share in the comments where exactly you encounter sodium tetraborate in practice. Good luck and good health to you!
Impact on the body
Sodium tetraborate is of low toxicity if consumed in food or if it comes into contact with the skin. However, it may cause corrosion if it comes into contact with eyes. Borax can also cause skin irritation. People who ingested sodium borate experienced nausea, vomiting, abdominal pain, and diarrhea. When inhaling borax, people complained of dry mouth, nose and throat. Some of the symptoms of overdose were cough, sore throat, shortness of breath and nosebleeds.
Children are more sensitive to the effects of borax than adults. Convulsions, confusion and coma have been reported in some children with sodium tetraborate poisoning. Sodium borate has a similar effect on animals. If ingested, the first signs of poisoning may appear within two hours.
What foods contain sodium tetraborate?
Products that contain borax can be liquid, granules, tablets, or powders. They are used in residential and commercial premises, hospitals and schools. More than five hundred products containing sodium borate are sold in the United States. Soil fertilizers, pesticides, household cleaners and detergents, laundry and personal care products.
Due to its antiseptic, antifungal, antiviral and antibacterial properties, it can be used in the treatment of cutaneous ringworm, for the disinfection of wounds and the treatment of otitis media.
What happens to sodium tetraborate when it enters the body?
Borax is rapidly absorbed when ingested with food or inhaled. It penetrates poorly upon immediate contact with the skin. But only if there is no damage to the skin. There is no evidence that sodium tetraborate breaks down naturally in the body. It can be stored in bones and adipose tissue for a long time. Most borax is excreted from the body in urine within four days of ingestion.
Has anyone studied the carcinogenic effects of long-term exposure to borax?
Studies with workers who inhaled borax over a long period of time found no negative effects of sodium tetraborate in the long term. However, they had constant complaints of vomiting, nausea, diarrhea and abdominal pain. Always follow the manufacturer's instructions on the product label!
Borax for the production of “lizuns” (smima)
Borax has a long history of use in household goods and to make slime toys, a sticky substance that is a huge hit with teenagers. This product is not safe for children due to the borax it contains, as well as solvents. You can make your own slime using cornstarch .
Borax and boric acid - what's the difference?
Borax and boric acid have different chemical compositions, so they are not the same thing, but in general both substances are dangerous because they have the same base: the chemical element boron.
It is better to replace this dangerous product with products that are much more harmless and just as effective, such as baking soda (for degreasing) or percarbonate of soda (for bleaching ).
Stone care
When using borax, you must take precautions: store in a closed container in a dry, dark place, use protective gloves when working and avoid contact with skin and mucous membranes.
Previous article Mineral brookite - properties, deposits and applications Next article Mineral vanadinite - properties, significance of the stone and applications.
Sources
- https://pressadv.ru/samodelkinu/bura-formula.html
- https://stroy-podskazka.ru/pajka/bura/
- https://ArmRinok.ru/obrabotka/bura-v.html
- https://m-strana.ru/articles/bura-formula/
- https://WikiMetall.ru/spravochnik/chto-takoe-bura.html
- https://kamneteka.com/bura-svoystva-znachenie-i-primenenie/
- https://pressadv.ru/stali/bura-tehnicheskaya.html
Storage
Disodium tetraborate should be stored in special rooms accessible only to persons who have undergone special instructions. Store in tightly closed containers that are properly labeled. Try to minimize dust in the room. Provide appropriate personal protective equipment near the premises.
Disodium tetraborate can be stored in paper, cardboard, polypropylene , or polyethylene .