CLASSIFICATION
Odontogenic infections (infections of the oral cavity), depending on the anatomical location, are divided into truly odontogenic
associated with damage to tooth tissue (caries, pulpitis);
periodontal
, associated with damage to the periodontium (periodontitis) and gums (gingivitis, pericoronitis), surrounding tissues (periosteum, bone, soft tissues of the face and neck, maxillary sinus, lymph nodes);
non-odontogenic
, associated with damage to the mucous membranes (stomatitis) and inflammation of the major salivary glands.
These types of infections can cause serious life-threatening complications from the cranial cavity, retropharyngeal, mediastinal and other localizations, as well as hematogenously disseminated lesions of the heart valve apparatus, sepsis.
Purulent infection of the face and neck
may be of non-odontogenic origin and includes folliculitis, furuncle, carbuncle, lymphadenitis, erysipelas, hematogenous osteomyelitis of the jaws.
Specific infections (actinomycosis, tuberculosis, syphilis, HIV) can also be observed in the maxillofacial area.
MAIN PATIENTS
Infections of the oral cavity are associated with the microflora that is constantly present here. Usually this is a mixed flora, including more than 3-5 microorganisms.
With truly odontogenic
infections, along with facultative bacteria, primarily viridans streptococci, in particular (
S.mutans, S.milleri
), anaerobic flora is released:
Peptostreptococcus
spp.,
Fusobacterium
spp.,
Actinomyces
spp.
In case of periodontal infection, five main pathogens are most often isolated: P.gingivalis, P.intermedia, E.corrodens, F.nucleatum, A.actinomycetemcomitans
, less commonly
Capnocytophaga
spp.
Depending on the location and severity of the infection, the patient’s age and concomitant pathology, changes in the microbial spectrum of pathogens are possible. Thus, severe purulent lesions are associated with facultative gram-negative flora ( Enterobacteriaceae
spp.) and
S.aureus
.
In elderly patients and hospitalized patients, Enterobacteriaceae
spp also predominate.
In the conditions of domestic bacteriological laboratories, it is quite difficult to isolate a specific pathogen of a certain odontogenic infection. However, it seems possible to localize pathogens that play a major role in the development of oral infections in supragingival and subgingival plaque.
PRINCIPLES OF TREATMENT OF INFECTIONS OF THE ORAL CAVITY AND MAXILLOFACIAL AREA
Treatment of odontogenic infection
often limited to local therapy, including standard dental procedures.
Systemic antibacterial therapy is carried out only when odontogenic infection spreads beyond the periodontal period (under the periosteum, into the bones, soft tissues of the face and neck), in the presence of elevated body temperature, regional lymphadenitis, and intoxication.
When choosing antimicrobial therapy in a hospital setting, in cases of severe purulent infection, it is necessary to take into account the possibility of the presence of resistant strains among anaerobes such as Prevotella
spp.,
F. nucleatum
to penicillin, which determines the prescription of broad-spectrum drugs, amoxicillin/clavulanate in monotherapy or a combination of fluoroquinolone with metronidazole.
Due to increasing levels of resistance by Streptococcus
spp.
to tetracycline and erythromycin, these drugs can only be used as alternatives. Since metronidazole has moderate activity against anaerobic cocci ( Peptostreptococcus
spp.), simultaneous administration of β-lactam antibiotics is necessary to increase effectiveness.
Preventive systemic use of AMPs when performing dental procedures in the oral cavity or periodontium does not guarantee a reduction in the incidence of infectious complications in somatically healthy patients. Convincing data on the sufficient effectiveness of topical antibiotics for oral infections have not been obtained. At the same time, oral bacteria are a reservoir of determinants of resistance to AMPs. Excessive and unjustified use of antibiotics contributes to their appearance and the development of resistance of pathogenic microflora.
ODONTOGENIC AND PERIODONTAL INFECTION
PULPITIS
Main pathogens
Viridans streptococci ( S. milleri
), non-spore-forming anaerobes:
Peptococcus
spp.,
Peptostreptococcus
spp.,
Actinomyces
spp.
Choice of antimicrobials
Antimicrobial therapy is indicated only in case of insufficient effectiveness of dental procedures or spread of infection into surrounding tissues (periodontal, periosteal, etc.).
Drugs of choice
: phenoxymethylpenicillin or penicillin (depending on the severity of the disease).
Alternative drugs
: aminopenicillins (amoxicillin, ampicillin), inhibitor-protected penicillins, cefaclor, clindamycin, erythromycin + metronidazole.
Duration of use
: depending on the severity of the current (at least 5 days).
PERIODONTITIS
Main pathogens
Microflora is rarely detected in the periodontal structure and is usually S.sanguis, S.oralis, Actinomyces
spp.
In periodontitis in adults, gram-negative anaerobes and spirochetes predominate. P.gingivalis, B.forsythus, A.actinomycetemcomitans and T.denticola
are highlighted most often.
In juveniles, there is rapid involvement of bone tissue in the process, with A. actinomycetemcomitans
and
Capnocytophaga
spp.
P.gingivalis
is rarely isolated.
In patients with leukemia and neutropenia after chemotherapy along with A. actinomycetemcomitans
C.micros
is isolated , and in prepubertal age -
Fusobacterium
spp.
Choice of antimicrobials
Drugs of choice
: doxycycline; amoxicillin/clavulanate.
Alternative drugs
: spiramycin + metronidazole, cefuroxime axetil, cefaclor + metronidazole.
Duration of therapy
: 5-7 days.
For patients with leukemia or neutropenia after chemotherapy, cefoperazone/sulbactam + aminoglycosides are used; piperacillin/tazobactam or ticarcillin/clavulanate + aminoglycosides; imipenem, meropenem.
Duration of therapy
: depending on the severity of the course, but not less than 10-14 days.
PERIOSTITIS AND OSTEOMYELITIS OF THE JAWS
Main pathogens
With the development of odontogenic periostitis and osteomyelitis, S. aureus
, as well as
Streptococcus
spp., and, as a rule, anaerobic flora prevails:
P.niger, Peptostreptococcus
spp.,
Bacteroides
spp.
Specific pathogens are less commonly identified: A.israelii, T.pallidum
.
Traumatic osteomyelitis is often caused by the presence of S.aureus
, as well as
Enterobacteriaceae
spp.,
P.aeruginosa
.
Choice of antimicrobials
Drugs of choice
: oxacillin, cefazolin, inhibitor-protected penicillins.
Alternative drugs
: lincosamides, cefuroxime.
When P.aeruginosa
, antipseudomonas drugs (ceftazidime, fluoroquinolones) are used.
Duration of therapy
: at least 4 weeks.
ODONTOGENIC MAXILLARY SINUSITIS
Main pathogens
The causative agents of odontogenic maxillary sinusitis are: non-spore-forming anaerobes - Peptostreptococcus
spp.,
Bacteroides
spp., as well as
H.influenzae, S.pneumoniae
, less often
S.intermedius, M.catarrhalis, S.pyogenes
.
Isolation of S.aureus
from the sinus is characteristic of nosocomial sinusitis.
Choice of antimicrobials
Drugs of choice
: amoxicillin/clavulanate. For nosocomial infection - vancomycin.
Alternative drugs
: cefuroxime axetil, co-trimoxazole, ciprofloxacin, chloramphenicol.
Duration of therapy
: 10 days.
Processing of dental instruments
Sanitary legislation puts forward strict requirements for the processing of dental instruments, which must be met by all dental offices without exception, regardless of their form of ownership. Such high demands are completely justified, because dental instruments can injure the oral mucosa, which is associated with a high risk of infection.
For the smooth operation of the dental office, it is necessary to have a sufficient number of sterile instruments, at least ten sets for each doctor per shift. This approach will allow us to serve a large number of patients and at the same time have time to systematically process the used instruments.
According to the requirements of sanitary legislation, dental instruments must be processed in three stages:
- Disinfection;
- Pre-sterilization treatment;
- Sterilization.
Disinfection of dental instruments
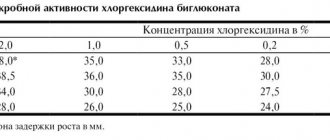
So, the first stage of processing instruments is disinfection, which is designed to minimize the level of contamination of instruments by microorganisms. Disinfection is carried out using disinfectants. The choice of disinfectant must be approached responsibly. The disinfectant, firstly, must effectively destroy microorganisms and, secondly, not spoil the instruments. These properties are fully met by disinfectants for dentistry “Septolite Tetra” and “Septolite Dental”.
Instruments are disinfected after each client. To do this, used instruments, opened and disassembled, are immersed in a container with a disinfectant. The liquid level should completely cover the instruments. Disinfection must be carried out according to the regimen used for viral infections.
At the end of the holding time, pre-sterilization cleaning begins, which is carried out to clean the instruments from protein contaminants. The products “Septolite Tetra” and “Septolite Dental” allow you to combine disinfection and PSO in one stage. Therefore, after disinfection is completed, the instruments are thoroughly cleaned using brushes directly in the container with the disinfectant.
Disinfection of impressions and prosthetic blanks is carried out before they are sent to the dental laboratory and before installation on the patient. After disinfection, the impressions and dentures are washed with distilled water. This completes the processing of these dental products.
Sterilization of instruments
All products that come into contact with blood and the wound surface, which can lead to injury to the mucous membrane, must be sterilized. Dental instruments are processed using steam or air sterilization. To do this, disinfected instruments are placed in packaging (paper bags or containers) and sent to a sterilizer. Sterilization in an autoclave is carried out for 20 minutes at a temperature of 132 °C, in a dry-heat oven for 60 minutes at a temperature of 180 °C.
Sterile instruments are stored packaged in Kraft bags, and unpacked in UV sterilizers. Please note that the assistant should place sterile instruments on the table immediately before starting the manipulation.
According to sanitary legislation, chemical sterilization using disinfectants is allowed only in cases where other sterilization methods cannot be used. This applies to the sterilization of instruments made from heat-labile materials. Chemical sterilization is carried out strictly in compliance with antiseptic rules. After chemical sterilization is completed, the instruments are washed with distilled water.
PURULENT INFECTION OF SOFT TISSUE OF THE FACE AND NECK
Main pathogens
Purulent odontogenic infection of the soft tissues of the face and neck, tissue of deep fascial spaces is associated with the release of polymicrobial flora: F. nucleatum
, pigmented
Bacteroides, Peptostreptococcus
spp.,
Actinomyces
spp.,
Streptococcus
spp.
ABSCESSES, PHLEGMONS OF THE FACE AND NECK
Main pathogens
With an abscess in the orbital area in adults, a mixed flora is released: Peptostreptococcus
spp.,
Bacteroides
spp.,
Enterobacteriaceae
spp.,
Veillonella
spp.,
Streptococcus
spp.,
Staphylococcus
spp.,
Eikenella
Streptococcus
spp.,
Staphylococcus
. predominate in children .
The causative agents of abscesses and phlegmon of non-odontogenic origin, most often caused by minor skin lesions, are S.aureus, S.pyogenes
.
With putrefactive-necrotic phlegmon of the floor of the mouth, a polymicrobial flora is released, including F.nucleatum, Bacteroides
spp.,
Peptostreptococcus
spp.,
Streptococcus
spp.,
Actinomyces
spp.
S.aureus
can be isolated in patients with severe disease (more often in patients suffering from diabetes and alcoholism).
Choice of antimicrobials
Drugs of choice:
inhibitor-protected penicillins (amoxicillin/clavulanate, ampicillin/sulbactam), cefoperazone/sulbactam.
When P.aeruginosa
- ceftazidime + aminoglycosides.
Alternative drugs:
penicillin or oxacillin + metronidazole, lincosamides + aminoglycosides of the II-III generation, carbapenems, vancomycin.
Duration of therapy:
at least 10-14 days.
BUCCAL CELLULITE
Main pathogens
Usually observed in children under 3-5 years of age. The main pathogen is H. influenzae
type B and
S. pneumoniae
.
In children under 2 years of age, H. influenzae
is the main pathogen, and bacteremia is usually observed.
Choice of antimicrobials
Drugs of choice
: amoxicillin/clavulanate, ampicillin/sulbactam, third generation cephalosporins (cefotaxime, ceftriaxone) IV, in high doses.
Alternative drugs
: chloramphenicol, co-trimoxazole.
Duration of therapy
: depending on the severity of the current, but not less than 7-10 days.
LYMPHADENITIS OF THE FACE AND NECK
Main pathogens
Regional lymphadenitis in the face and neck is observed with infection in the oral cavity and face. Localization of lymphadenitis in the submandibular region, along the anterior and posterior surfaces of the neck in children aged 1-4 years, is usually associated with a viral infection.
Abscess formation of lymph nodes is usually caused by a bacterial infection. With unilateral lymphadenitis on the lateral surface of the neck in children over 4 years of age, GABHS and S.aureus
.
Anaerobic pathogens, such as Bacteroides
spp.,
Peptococcus
spp.,
Peptostreptococcus
spp.,
F.nucleatum, P.acnes
, can cause the development of odontogenic lymphadenitis or inflammatory diseases of the oral mucosa (gingivitis, stomatitis), cellulite.
Choice of antimicrobials
Drugs of choice: AMPs corresponding to the etiology of the primary source of infection.
The extreme importance of infectious pathology in the modern world, with its high mobility and economic interdependence, lies in its global danger to the public health of each individual country and the entire world community as a whole. A comprehensive assessment of the risks to public health caused by infectious agents conducted by WHO international experts led to the conclusion that the current situation in the world is far from stable. Population growth, rapid urbanization and globalization, environmental degradation, the rapid introduction of new forms of health care, and the unjustifiable large-scale use of antimicrobial drugs have led to disruption of the delicate balance in the microbiological community. At the turn of the 20th-21st centuries, we witnessed unprecedented rates in history of the emergence of “new” diseases (at least 1 nosology per year!), the spread of infectious diseases with atypical clinical manifestations (25-57%) and “returning” infections (diphtheria, tuberculosis , syphilis, etc.), which, of course, determined the severity of the problem of infectious safety in the modern world [2, 22].
It is our firm belief that a solution to this problem can only be found jointly with the involvement of specialists from different fields of knowledge. After all, the essence of infectious pathology as a medical problem lies not only in the risk of epidemics and the aggravation of the course of acute infectious diseases. Today it is no secret that long-term persistent infections with scanty clinical manifestations are coming to the fore, the pathogens of which play an etiological or trigger role in the development of hematological, oncological, neurological, dental diseases, kidney damage, liver, joints, etc. [7, 8, 12, 15-18, 21, 23]. The need for a deep understanding of the issues of infectious pathology and infectious safety by doctors of various specializations is an urgent, vitally important problem.
From this point of view, dentists occupy a special niche, given the prevalence of dental diseases among people of all age groups (56-99% of the population) and the massive number of people seeking dental care. The unique quantitative and qualitative composition of the oral microflora and the intensification of modern dentistry extremely increase the risk of cross-infection of patients and professional infection of medical workers. Achieving sanitary and epidemiological well-being in medical dental institutions at the outpatient and hospital level can only be achieved by combining the efforts of dentists and infectious disease specialists in the study of infection-associated pathology. This is consistent with the statement of the American Dental Association (2012): “Dental care is an integral component of general health care. By improving the quality of dental services, we improve the public health and well-being of the nation.”
Currently, the proportion of people with a burdened premorbid background among dental patients reaches 93.3%. The most common among them are people with infectious pathologies (acute and chronic purulent-septic diseases, viral hepatitis, herpes virus infection, HIV infection, mycoses, etc.), food and drug allergies, often suffering from acute respiratory viral infections (ARVI), etc. Moreover, in most cases, patients do not even suspect that they have a particular disease, and in the time pressure of a dental appointment, it is extremely difficult for a doctor to obtain comprehensive information about the patient’s health status. Therefore, any patient who seeks dental care should be considered by a dentist as posing an epidemiological danger, regardless of the degree of risk of infection!
According to participants at the WHO Informal Network on Infection Prevention and Control in Health (2008), health care-associated infections (HAIs) represent a major public health problem and a significant burden for patients and health care workers, affecting all countries [6 ]. HAI refers to a hospital-acquired infection (HAI), which the WHO European Office defines as “any clinically significant infectious disease that affects a patient as a result of his admission to a health care facility or seeking medical care or employees of a health care facility as a result of their work in this institution outside depending on the appearance of symptoms of the disease during or after a stay in a medical facility” [16].
Of the main groups of nosocomial infections in dentistry, the most relevant are infections of surgical wounds and bloodstream [6], which develop as a result of a blood-contact transmission mechanism (through medical procedures) or a contact mechanism in which pathogens are localized on the skin and its appendages, the mucous membrane of the eyes, and the oral cavity. , the wound surfaces of one patient, infect another patient through direct contact or through contaminated objects in the hospital environment. It is necessary to remember the possibility of cross-infection of patients and professional infection of medical personnel as a result of the implementation of airborne droplets and contact mechanisms of infection transmission. The probable routes of transmission of infection from patient to patient during dental interventions are presented in the form of an epidemiological chain (Fig. 1, see color plate)
.
Rice.
1. Possible routes of transmission of infection from patient to patient during dental procedures. Modern factors contributing to the growth of nosocomial infections in dental practice include:
— the current epidemiological situation regarding HIV infection, viral hepatitis, herpes virus infections, etc.;
— distribution of consumption of parenteral psychotropic drugs;
— rapid growth of resistance of microorganisms to antimicrobial drugs up to multidrug resistance (for example, M. tuberculosis
, gram-negative bacteria);
— the spread of resistant strains among patients of a medical institution in the absence of effective infection control programs;
— widespread use of invasive methods of diagnosis and treatment;
— creation of large high-tech medical complexes with a specific microecology;
— difficulties in disinfecting and sterilizing complex medical equipment;
— unfavorable environmental situation;
— an increase in the number of patients with manifestations of secondary immunodeficiency.
Exogenous (primary) and endogenous (secondary) sources of infection during dental procedures are described. With exogenous infection, the source of infection is patients and medical personnel infected with pathogenic and opportunistic microorganisms. Endogenous infection should be understood as activation of latent infection under the influence of stress (fear, exacerbation of chronic diseases, acute respiratory viral infections, etc.) and autoinfection, the etiological factors of which in 85% of cases are representatives of opportunistic flora [1, 4].
Endogenous infection deserves special attention, the ease of which is explained by the extremely rich auchthonous and allochthonous microflora of the mucosa, which varies significantly from person to person, as well as from one and the same person at different times. Representatives of the authentic microflora are resident microorganisms (from 160 to 300 species; up to 100 species can be isolated from 1 site, of which at least 30 species are permanently living) and transient microorganisms, the composition of which depends on environmental factors (consumed products and water, hygiene procedures, etc.). Representatives of the allochthonous flora enter the oral cavity from other microbiocenoses (from the intestines, nasopharynx, respiratory tract, etc.). As a result of research conducted in 2003, L.P. Zueva et al., it was shown that more than 1/3 of patients are carriers of Staphylococcus aureus
, 10% of strains of which are resistant to 5 or more antibacterial drugs
(Fig. 2, see color insert)
.
Rice. 2. Representatives of the microflora of the oral cavity, nose and pharynx, identified in patients who sought dental care in the department of maxillofacial surgery. According to the results of long-term studies by J. Prieto-Prieto, A. Calvo and T. Kuriyama et al., in the spectrum of odontogenic bacteria, Peptostreptococcus, Prevotella
and
Fusobacterium
, and Str. among aerobes
. viridians (Fig. 3, see color insert)
Rice.
3. Spectrum of odontogenic bacteria (Kuriyama et al., 2002; Prieto-Prieto J., Calvo A., 2004). [13, 19]. According to A.A. Zorkina et al. (2009), most often in inflammatory processes in the oral cavity, Actinobacillus actinomycetemcomitans
and
Porphyromonas gingivalis
, less often -
Bacteroides forsythus, Prevotella intermedia, Prevotella nigrenscens, Fusobacterium
spp.,
Peptococcus micros, Carnocytophaga
spp.,
Treponema denticola, Treponema sokranskii
.
Typical causative agents of odontogenic infections are Peptostreptococcus
spp.,
Peptococcus
spp.,
Fusobacterium
spp., periodontal infections are
A. actinomycetemcomitans, P. gingivalis, Pr.
intermedia, Eikenella corrodens, Fusobacterium nucleatum (especially in severe infections).
It is important for practicing physicians to remember that the diversity of microorganisms creates unique opportunities for the transmission of virulence and resistance determinants, the reservoir of which is the normal microflora [9, 20]. The possibility of exchanging genetic information between bacteria of the genitourinary tract and oral cavity, and in laboratory conditions, between taxonomically distant species of microorganisms has been described [9].
Infectious agents of oral tissues, from commesals to pathogens, play an etiological role not only in the initiation of dental diseases (caries, periodontitis, gingivitis, etc.). The integrity of the soft tissue barrier of the oral cavity, disrupted during dental procedures, injuries, and infections, leads to transient bacteremia (fungemia, viremia) and the entry of microorganisms into tissues that are not characteristic of them (for example, connective tissue, blood), as a result of which systemic changes in gene expression are induced, leading to to increase the virulence of infectious agents. Becoming endogenous pathogens, microorganisms can play a trigger role in the formation of pathologies of the digestive, respiratory, cardiovascular (DIC syndrome, infective endocarditis, atherosclerosis) systems, rheumatism, nephropathy, many infectious and allergic conditions, autoimmune diseases (rheumatoid arthritis - RA, Behçet and others) [10, 11, 20]. Thus, it has been proven that in S. sanguinis, S. oralis, S. mutans, S. salivarius
The constitutive expression of virulence genes is sufficient to induce fibrinogen binding by platelets, aggregation and thrombus formation when entering the bloodstream, and when deposited on altered or abnormally developed valves, lead to the development of infective endocarditis.
Changes in virulence can occur under the influence of genes regulated by the acidity of the environment into which microorganisms enter. Thus, with an increase in pH to 7.3-7.5, thrombin-like activity increases in S. gordonii
.
Upon contact with laminin and collagen of damaged heart valves, S. gordonii
triggers the expression of laminin-binding protein, which increases the adhesion of bacteria to the valve surface [11].
An interesting phenomenon is bacterial mimicry in representatives of the oral microflora. S. sanguinis cell wall protein epitope
- PAAP, associated with platelet aggregation, with an arthritic epitope of type II collagen, may be one of the causes of RA development.
The heat shock protein (HSP60) normally produced in humans is highly homologous to the bacterial HSPs of Mycobacterium
(HSP65),
S. sanguinis
(HSP65), and
Helicobacter pylori
(HSP60). The resulting antibodies cross-react with the development of a systemic inflammatory reaction and, through activation of the complement system, lead to immune inflammation in the vascular wall, arthritis, and Behçet's disease [14].
Infectious diseases, the source of which can be carriers of “classical” pathogens (hepatitis, HIV infection, tuberculosis, syphilis, herpes viruses, etc.), account for no more than 15% of all nosocomial infections in dental medical institutions. According to WHO (2007, 2010) [2, 6], among all infectious diseases, the most relevant for dentists and their patients are viral hepatitis A, B, C, D (TTV, G, SEN - ?), HIV infection, herpes virus infection , tuberculosis, candidiasis, acute respiratory viral infections and influenza, measles, legionellosis, mumps, streptococcal diseases (tonsillitis, scarlet fever), staphylococcal diseases (sore throat, pneumonia, enterocolitis, purulent lesions of the skin and subcutaneous tissue, etc.), diphtheria, syphilis, gonorrhea and tetanus . The main infectious diseases with clinical manifestations in the oral cavity are presented in the table
.
It should be recognized that the manifestations of even well-known infectious diseases can change against the background of the patient’s chronic diseases or during co-infection, acquiring features that are unusual for them, which complicates their clinical diagnosis and requires timely laboratory examination.
In addition to those presented in the table
infectious diseases, there is a large group of viral infectious diseases that do not have characteristic pathological manifestations in the oral cavity, but are transmitted by blood contact during dental interventions, which leads to the development of life-threatening diseases for patients and medical personnel (viral hepatitis B, C, D; cytomegalovirus infection; Epstein -Barr virus infection - except for infectious mononucleosis and herpes zoster described above; herpes virus infection caused by HHV-6, HHV-7, HHV-8 type, etc.).
All of the above indicates the great role of infectious agents in human pathology in general and in dental practice in particular. A dentist can meet with an infectious patient at any time. The life of the patient and the timely suppression of the epidemic process often depend on his erudition and knowledge, which makes it possible to prevent cross-contamination and infection of medical personnel when providing dental care.
NON-ODONTOGENIC AND SPECIFIC INFECTION
NECROTIC STOMATITIS (VINCINS ULCER-NECROTIC GINGIVOSTOMATITIS)
Main pathogens
Fusobacterium is concentrated in the gingival sulcus
, pigmented
Bacteroides
, anaerobic spirochetes. With necrotizing stomatitis, there is a tendency for infection to quickly spread into surrounding tissues.
The causative agents are F. nucleatum, T. vinsentii, P. melaninogenica, P. gingivalis and P. intermedia
.
In patients with AIDS, C. rectus
.
Choice of antimicrobials
Drugs of choice:
phenoxymethylpenicillin, penicillin.
Alternative drugs:
macrolide + metronidazole.
Duration of therapy:
depending on the severity of the current.
ACTINOMYCOSIS
Main pathogens
The main causative agent of actinomycosis is A.israelii
, association with gram-negative bacteria
A. actinomycetemcomitans
and
H. aphrophilus
, which are resistant to penicillin but sensitive to tetracyclines, is also possible.
Choice of antimicrobials
Drugs of choice
: penicillin at a dose of 18-24 million units/day, with positive dynamics - transition to step-down therapy (phenoxymethylpenicillin 2 g/day or amoxicillin 3-4 g/day).
Alternative drugs
: doxycycline 0.2 g/day, oral drugs - tetracycline 3 g/day, erythromycin 2 g/day.
Duration of therapy
: penicillin 3-6 weeks IV, drugs for oral administration - 6-12 months.