Caries is a common dental disease that destroys the hard tissues of teeth. According to statistics, it affects about 95-98% of the population of economically developed countries. In the treatment of caries, medicinal pads can be used. In what cases and for what they are used, we will tell you in this article.
In this article
- What is a therapeutic pad in dentistry?
- In what cases is a lining used for caries?
- Why are medicinal pads used in the treatment of deep caries?
- How is a therapeutic napkin installed for deep caries?
- Materials of therapeutic pads
- How do combined pastes work?
- Pros and cons of using medicinal pads in dentistry
In what cases is a lining used for caries?
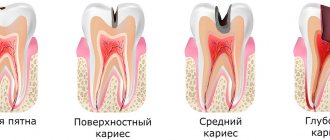
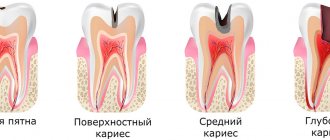
Caries is a slowly progressive pathological process that goes through several stages. At an early stage, a carious lesion looks like a light spot on the enamel, while there is still no hole or pain in the tooth. As the disease progresses, the spot turns into a carious cavity. At the stage of superficial caries, this cavity is located within the tooth enamel; with medium and deep caries, it affects dentin tissue.
For caries in the spot stage and the superficial form of the disease, pads with a therapeutic effect are not needed; for average caries, they can be used according to indications. Most often, medicinal dental pads are used for deep caries. The peculiarity of deep caries is that the carious cavity is located as close as possible to the pulp - the neurovascular bundle, or dental nerve. At this stage, treatment is quite painful and there is a high risk of complications in the form of pulpitis and periodontitis.
Dental linings help increase the effectiveness of treatment of deep caries.
Insulating pads
When treating teeth, be it caries, pulpitis or dental prosthetics, certain filling materials are used.
For example, insulating or therapeutic pads, which are used to treat pulpitis, isolate the nearest pulp wall from the main filling for better distribution of chewing pressure, prevent microcracks, and reduce stress associated with the use of composite materials.
Insulating pads must be durable, impermeable, non-toxic, have thermal insulating properties, do not shrink, be clearly visible on x-rays, and have properties close to the physical properties of tooth tissue. They must fit well with the composite system and have adhesion to the tooth tissues. Some pads may also have medicinal properties, releasing substances that have a healing effect.
Why are medicinal pads used in the treatment of deep caries?
The purpose of a healing pad is to protect the pulp. After the dentist has removed the tissue affected by caries, cleaned and prepared the carious cavity for filling, a very thin layer of dentin, the bone tissue of the tooth, remains between it and the dental pulp. This creates a high risk of microbes entering the dental nerve and developing pulpitis. To prevent the spread of infection into the pulp, stop the inflammatory process and stimulate the production of a new layer of dentin, a special gasket is applied.
When a gasket is temporarily applied, the entire carious cavity is covered with it and a temporary filling is installed for a period of two weeks to several months. Permanent linings for deep caries are applied pointwise in the area of the tubercles of the neurovascular bundle. In the second case, along with the therapeutic one, an insulating gasket is installed.
Requirements for insulating gaskets
The insulating gasket must:
- Protect dentin from chemical and temperature irritants;
- Easy to install;
- Withstand the most aggressive effects of saliva if the integrity of the filling is compromised;
- Withstand the maximum possible chewing load;
- Be bonded to the tooth more strongly than to the filling;
- Do not have a negative effect on the dental pulp.
Also, the insulating lining should not change the natural color of the tooth enamel.
How is a therapeutic napkin installed for deep caries?
Depending on the form and complexity of the disease, as well as the chosen method of caries treatment, the dentist can install the lining by direct or indirect application. The first option assumes that it is applied directly to the pulp area, ensuring direct contact between the gasket and the dental nerve. The method is used for tooth injuries with exposure of the nerve, for fibrous pulpitis. The second method is to apply a lining using dentinal tubules without direct contact with the pulp. It is often chosen for the treatment of deep stage caries and acute pulpitis.
Scope of application
There are many types of treatment in which a doctor can use GIC, and one or another type of glass polyalkinate is suitable for each:
- for filling teeth with treated caries: mainly used for those teeth that do not experience chewing load. But in modern dentistry, types of glass ionomer cement have already appeared that are close in strength to composite fillings,
- for fixation of crowns, bridges, prostheses and other orthopedic structures: in this area, a modified GIC is used, which hardens faster than the classic one,
- as a lining (insulating) material when installing composite fillings1,
- for dental restoration, including in pediatric dentistry,
- for filling baby teeth,
- for sealing fissures.
Materials of therapeutic pads
Several types of dental dams are used in the treatment of deep caries:
- varnishes, solutions, light-curing cements and polymers based on calcium hydroxide;
- zinc-eugenol and combined pastes.
Preparations based on calcium hydroxide have a powerful bactericidal effect. The application of these compounds to the bottom of the carious cavity prevents the penetration of microbes into the dental pulp and the development of the inflammatory process. Calcium hydroxide also promotes the production of new bone tissue in the area between the cavity and the nerve of the tooth.
Aqueous suspensions based on calcium hydroxide are usually applied in a thin layer to the bottom of the carious cavity, dried and a temporary filling is placed on top. They are replaced after 30-45 days. Also, in the treatment of dental caries, chemically cured cements are often used. They are pastes containing ether and hydroxide in a 1:1 ratio. These pastes harden directly in the patient's mouth and have excellent insulating properties in relation to fillings. Light-curing polymers must be used very carefully, because there is a risk of burning the dental nerve. At the same time, they have very high strength and, in the hands of a professional dentist, can be an effective means of treating caries. Zinc-eugenol pastes are widely used as an antiseptic for temporary fillings.
How to fill with glass ionomer cement
The technique of preparing the crown surface for the application of GIC is similar to the technique of filling with a conventional composite, but there are some nuances. Since glass ionomers have very high chemical adhesion, the tooth cavity does not need to be deeply prepared with a drill. It is enough to remove the top layer and treat the cavity with polyacrylic acid. In some ways, this process is reminiscent of applying a primer before painting walls. Next, the surface is washed and thoroughly dried.
Mixing is done either manually, if the GIC is powdery, or in a special capsule. The density of the mass depends on the purpose of its use. If we are talking about closing fissures, then the consistency of the batch should be similar to sour cream. To seal or install insulating gaskets, the mass is kneaded more thickly.
The process of introducing GIC into the cavity depends on the mixing method. If the doctor prepared the material manually, then it is applied with special plastic tools, but if the cement was mixed mechanically, then it is loaded into the desired area with a special gun. When filling the chewing surface, its correct contour is created using a matrix, which is fixed by dental pressure. Special matrices have been developed for cervical filling.
During this manipulation, the doctor makes sure that no moisture (for example, saliva) gets into the cement, otherwise dehydration will begin inside the filling, which can disrupt the structure and deteriorate the quality characteristics of the material. Therefore, after completing the modeling of the contact surface, a water-repellent coating is applied to the glass ionomer filling.
The final processing of GIC (removal of excess material and polishing) is carried out after a day, or better yet, two. This happens because, firstly, the complete “ripening” of the filling lasts at least 24 hours. Secondly, during grinding the surface is heated by the rotating tool, which often leads to dehydration of the GIC. And even after the filling material has completely hardened when polishing it, glass ionomer manufacturers advise lubricating the surfaces of the abrasive discs with Vaseline.
How do combined pastes work?
The composition of combined pastes includes oils, fillers (zinc oxide or white clay), as well as medications. The therapeutic effect of a particular pad directly depends on the latter:
- Stimulating dentin growth and promoting enamel remineralization. These drugs are called odontotropic, they are based on fluorides, collagen, calcium glycerophosphate and other substances. They are used when it is necessary to increase the layer of bone tissue between the pulp and a deep carious cavity.
- Possessing anti-edematous and anti-inflammatory effects. They help stop the inflammatory process in the dental pulp; they are based on prednisolone and hydrocortisone. Typically, such preparations are temporarily placed at the bottom of the cavity and covered with a bandage on top. If the dentin layer next to the neurovascular bundle grows too slowly, then a spacer with an odontotropic effect is applied.
- Pads with an antiseptic effect based on metronidazole or chlorhexidine disinfect and prevent the growth of bacteria.
- Proteolytic enzymes contained in the drug are needed in the treatment of acute focal pulpitis, as well as in the treatment of deep caries.
Most combined pastes harden poorly and have low strength, so they are used as a temporary therapeutic material. After their removal, a different type of therapeutic pad is usually used.
A few words about the shortcomings
Nevertheless, despite such obvious advantages of the material, doctors also note some of its disadvantages:
- long-term hardening of the material: if the primary density appears 3-5 minutes after mixing, then the filling “ripes” completely only after a day, which increases the risk of destruction of its properties if the patient does not follow the doctor’s recommendations. For example, he begins to chew on a treated tooth,
- GIC is less durable than its composite analogues, so for now in dentistry it is not used as a full-fledged filling material, but as an auxiliary or temporary one.
- not very suitable for aesthetic dentistry because it has low transparency, poor color range and does not polish well.
Pros and cons of using medicinal pads in dentistry
The use of medicinal pads in dental practice has a number of advantages:
- They relieve inflammation, promote tissue regeneration, and help prepare the tooth for filling.
- Many drugs contain anesthetics that provide an analgesic effect, which is important when treating tissues near the sensitive dental nerve.
- Most materials are highly plastic, harden quickly and prevent the formation of microscopic cracks in the tooth.
In addition to the advantages, therapeutic pads have a number of disadvantages:
- They impair the adhesion of the filling to the walls and bottom of the cavity, which can shorten the service life of the restored tooth.
- If the technology for installing a medical pad is violated, the risk of tissue infection increases.
- Most materials dissolve when exposed to liquids, so they must be replaced frequently.
Despite the disadvantages, medicinal pads are still widely used in dentistry, as they have a wide spectrum of action and help solve many therapeutic problems.
What is glass ionomer cement (GIC) made of?
Glass ionomers are a chemical “commonwealth” of silicate and polyacrylic materials, which are becoming increasingly popular in the field of dental fillings, displacing classical cements made from zinc phosphates and zinc polycarboxylates.
Glass ionomer cements, also called glass polyalkynates, are a powder of calcium aluminosilicate glass into which fluorides are mixed. This granular mixture is combined with a liquid, which is polycarbonate acid. The resulting mass is used as a fastening, filling or restoration material.
Modern aspects of the use of gaskets and adhesive systems (Part 1)
In 1885, Chapin Harris published an article in which he recommended using special materials (asbestos, gutta-percha, a thin layer of cork, etc.) when using metal fillings to prevent thermal damage to the pulp. Subsequently, these materials were replaced by phosphate, polycarboxylate, glass ionomer cements, calcium hydroxide and were called lining materials, which, in turn, were divided into isolating and therapeutic. In the process of their use, many disagreements arose, and in some cases, an erroneous, in my opinion, stereotype of their use was developed, which is still difficult to change. This is especially noticeable when you read modern dental publications, where the advisability of using spacers is still considered as an opportunity to prevent the toxic effects of permanent filling materials on the dental pulp, as well as a means of inversely isolating the restoration from the influence of dental lymph. That is why the authors of these publications recommend placing an intermediate layer - a spacer - between the main filling material and dentin. In this case, the very name “insulating gasket” indicates the separating function of these materials. Along with this, spacers occupy space where, in fact, the means of binding the filling material to the dentin of the tooth (adhesive systems) should be located. It is this circumstance that has become the cause of many complications encountered when using gaskets, since none of them has sufficient adhesion force to dentin, comparable in the bonding force of permanent filling materials and adhesive systems to tooth tissue. This fact excludes the possibility of using spacers as bonding agents, which undoubtedly complicates adhesive measures and weakens the restoration. Considering the list of requirements for materials for gaskets, you can see that in fact these are the requirements for a perfect filling material, so if such a material is ever created, the need for gaskets will simply disappear, because Clinicians will now be able to use universal material for permanent fillings to restore teeth. It is necessary to finally understand that the use of gaskets is associated with the lack of an “ideal” filling material, because the gasket is the weak link in the restoration, because it does not increase its strength, but, on the contrary, reduces it. I would like to share my thoughts on this problem, however, first of all, it is necessary to find out what, in fact, we want to protect from the effects of permanent filling materials. In this case, the main object of protection is the dental pulp, without understanding the structural and functional properties of which it is impossible to consider these issues. It is a unique example of connective tissue that is almost completely surrounded by hard tissue, dentin, which limits the ability of the pulp to expand, thereby reducing its ability to tolerate swelling. In addition, it is the only organ capable of producing reparative dentin to protect against damage. High vitality of the pulp is ensured by: additional sources of tooth nutrition - anastomoses, anastomoses, annular circulatory system in the coronal pulp; loose connective tissue surrounding the vessels near the apical foramen, which eliminates the possibility of squeezing them during inflammation; abundant capillary network of the coronal pulp; cells of the reticuloendothelial system and hyaluronic acid, which are an important factor in protecting the structural formations of the pulp from harmful influences; stability of the enzyme inhibitor system. In essence, pulp and dentin are a single formation, where the state of the pulp is related to what happens in the dentin and vice versa. Since odontoblasts are responsible for dentinogenesis, they are the most characteristic cells of this complex. The processes of odontoblasts penetrate the dentinal tubules and form a single functional system – the dentinal-pulp complex (Fig. 1).
Fig.1. The structure of the dentin-pulp complex (Trowbridge, 1982).
Of no small importance in this system is given to peritubular dentin, which directly surrounds each dentinal tubule and forms its wall, and intertubular dentin is located between the dentinal tubules, making up the bulk of the peripulpar dentin (Fig. 2). Compared to intertubular dentin, peritubular dentin is characterized by a higher mineral content. It is as a result of the deposition of peritubular dentin that a gradual narrowing and obliteration of dentinal tubules occurs, resulting in the formation of sclerotic and replacement dentin, which are protective and adaptive zones of the carious process. Often, in parallel with the formation of these zones, the cellular elements of the pulp are replaced by fibrous connective tissue, which in this case is also considered a protective-adaptive reaction. In the absence of protective zones and the presence of a thin layer of dentin above the pulp, wide dentinal tubules and extensive zones of demineralization, histological manifestation of inflammation occurs.
Fig.2. Diagram of the structure of peritubular and intertubular dentin (Trowbridge, 1982).
The fact that the pulp is enclosed in an isolated cavity makes it not only practically inaccessible for inspection, but also complicates treatment. When it is objectively discovered, it is usually not possible to preserve it. However, even when the inflammation subsides, the doctor is still not sure that the process has not turned into acute and chronic inflammation in various areas of the pulp, in the equilibrium of which the exacerbation of the process may not occur for quite a long time. In such a state, an almost inflamed pulp can function for a long time under a filling with a medical pad, without returning to its original state, however, when and as a result of which this balance will be disrupted (exacerbation of the process), modern science is still powerless to determine. On this basis, in controversial situations, for example, In teeth used to support a bridge, it is advisable to depulpate them. It has long been believed that the main source of damage and ultimately necrosis of the pulp is the toxic effects of the monomers of composite resins, therefore it was strongly recommended that all composite fillings be carefully insulated with cement. However, in studies that tested composite materials, a very interesting fact was discovered, namely, that the composite materials were not so toxic as to cause inflammation of the pulp, but rather that the primary pulp response to the composites decreased over time until it disappeared completely. The results of many years of clinical observations allowed Brannstrom to conclude that the cytotoxic effect initiated by the application, for example, of silicate or composite materials directly on the peripulpal dentin did not differ significantly from those cases when the latter was not isolated with spacers. Moreover, the acids themselves, which are part of some filling materials, materials began to be used for etching enamel and dentin. From that moment on, the seemingly indisputable theory of “composite resin toxicity” as the cause of pulp inflammation collapsed. Scientists came to the conclusion that pulp damage caused by the toxic effects of composite materials is usually insignificant and are temporary and reversible, and in some cases are completely absent. In addition, it has been proven that with a thickness of the “dentinal bridge” of 0.5 mm, the pathological effect on the pulp is reduced by 75%, and with a thickness of 1 mm – by 90 %. Therefore, the best “spacers” are actually sclerotic and replacement dentin . In this regard, the statement of Sir John Tomes is instructive, who, back in 1859, stated that “it is better to leave a layer of pigmented dentin to protect the pulp than to run the risk of sacrificing the tooth.” The main cause of pulp damage that develops as a result of restorations is the waste products of bacteria that penetrate into the pulp due to a violation of the marginal fit of the filling material. This is usually associated with polymerization shrinkage of these materials, when marginal cracks appear in various areas of the restoration (Fig. 3 ).The essence of this phenomenon is that the curing of filling materials occurs as a result of a polymerization reaction, when the material passes from a liquid to a solid state. In this case, a significant change in its volume occurs due to a decrease in the distance between the molecules and, as a consequence, compression of the material. As a result of polymerization shrinkage, tension arises ( polymerization stress ), leading to “tightening” of the walls of the cavity, cohesive rupture of the hybrid layer and the emergence of negative pressure, causing fluid movement in the dentinal tubules with subsequent tension of the processes of odontoblasts and the death of the latter. In addition, many authors indicate on the possibility of a piezoelectric charge arising as a result of polymerization stress, which has a negative effect on the pulp. It is because of these complications that polymerization shrinkage is the main cause of postoperative sensitivity, relapse of caries, pulp necrosis, etc.
Fig.3. Three types of marginal fissure occurrence (Trowbridge, 1998). 1. Between dentin and smear layer. 2. Between the lubricated layer and the gasket. 3. Between the gasket and the restoration material.
Numerous clinical studies have proven a direct connection between pulp inflammation and filling leaks. It turned out that the ideal tightness of the dentinal tubules is extremely important for the pulp, because any movement of fluid in them causes irritation of the nerve fibers and is accompanied by pain. As is known, dentinal fluid in the tubules is retained under the action of capillary forces, however, physical, chemical or osmotic irritations cause its movement. This movement can occur as a result of drying out the cavity with a vacuum cleaner, irritation with heat or cold, the use of osmotically active substances, for example, sugar, etc.. Consequently, tooth hypersensitivity depends on the movement of moisture in the dentinal tubules and is in no way associated with the absence of a lining, which supposedly protects tooth tissue from various irritants . Dangerous for the pulp is not only the communication with the external environment, but also the movement of fluid in the dentinal tubules in the presence of a gap, which is not even always connected with the oral cavity. This happens, for example, when restoring class I cavities with composites, when the marginal seal is preserved and the gap is formed deeper. Under chewing load, the filling begins to “spring” and at the same time acts as a membrane pump ( pumping effect ). The movement of fluid damages the odontoblasts and irritates the subodontoblastic layer of the pulp, where numerous nerve endings are located, as a result of which the patient feels acute pain. The resulting pathological processes not only cause postoperative sensitivity, but over time can lead to the death of the pulp. The most effective technique to prevent the occurrence of increased tooth sensitivity is sealing the dentinal tubules with an adhesive system. However, in some cases, such as when filling a cavity with amalgam, sensitivity may disappear regardless of whether adhesive systems were used or not. It is assumed that the reason for this is the blocking of crevices and entrances to the dentinal tubules by amalgam corrosion products. Perhaps this is the only case when corrosion plays a “positive” role in nature. For this reason, it is always worth giving serious consideration to whether adhesive systems and spacers should be used before using an amalgam filling. The only situation when a lining under an amalgam is indicated is a deep carious cavity, in which the “dentinal bridge” must be covered with a therapeutic and insulating lining for the purpose of artificial formation of replacement dentin. In all other cases, when the walls and bottom of the carious cavity are quite dense, as a result of the formation of protective-adaptive zones, it is advisable to fill the cavity directly with amalgam or use adhesive systems as bonding agents.
ATTENTION! Any copying and placement in third-party sources of materials published on the website WWW.STOMPORT.RU is possible only if you provide an ACTIVE link to the source.
Treatment prognosis
With the right professional approach, it is possible to save the tooth, prevent pulpitis and periodontitis, and eliminate the need for tooth extraction. Recovery is possible even with severe destruction of the coronal part. If it is completely destroyed, it is possible to install an artificial crown, preserving the aesthetics and functionality of the tooth. If you do not resort to dental care in time, there is a high probability of pulp damage, further severe destruction, inflammation of the periodontal tissues - periodontitis, and complete tooth loss.