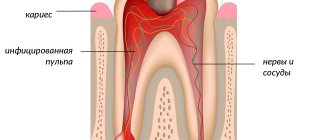
In this article we will look at Stanton-Capdepont syndrome, a disease determined at the gene level. It manifests itself as damage to the enamel and dentin, which results in not only an aesthetic defect, but also a subsequent decrease in the height of the bite. The size and shape of teeth are not affected during eruption.
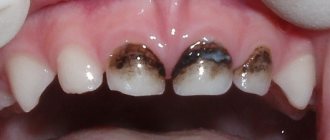
Discoloration is characteristic - staining the enamel grayish or brown. A medical history test, physical examination data, X-ray examination and EDI can correctly diagnose the syndrome. Its treatment usually consists of complex remineralizing therapy and prosthetics. Let's look at this syndrome in more detail.
Goals of the center:
- improving the quality of long-term observation, planned treatment and rehabilitation of patients who have suffered viral infections, including COVID-19, and who have complications of an immunopathological nature,
- exchange of scientific and clinical experience in the above area with Russian and foreign colleagues,
- diagnosis and rehabilitation of the consequences of autoimmune diseases.
Objectives of the Center
- Using a synergistic effect when combining the scientific and clinical potential of the specified departments of St. Petersburg State University and specialists in various fields (cardiorheumatology, pulmonology, neurology, gastroenterology, endocrinology, immunology and allergology, psychiatry, epidemiology) - to clarify the etiology, pathogenesis and development of effective methods for the treatment and prevention of health disorders in persons who have had a new coronavirus infection.
- Development of domestic and international cooperation in biomedical and clinical research of post-infectious autoimmune and immunopathological syndromes and diseases.
- Development of an individualized treatment plan for each patient in a short time within the framework of outpatient consultations, a clinical examination by a team of specialists and a one-day hospital stay.
- Creation of a telemedicine consultation center to work with patients outside St. Petersburg.
Post-Covid syndrome
is a condition that occurs in the form of long-term pathological symptoms after a new coronavirus infection, persisting for three months or more.
Causes of pathology
Stanton-Capdepont syndrome is a hereditary disease that occurs as a result of a mutation in the gene that is responsible for a specific matrix protein. It is transmitted in 50% of cases, both in girls and boys. The disease affects not only temporary teeth, but also permanent teeth.
The cause of irreversible changes is a decrease in the thickness of the enamel layer (enamel and dentin dysplasia), which disrupts the connections between the hard tissues of the tooth. Usually there is no predentin layer. Dentinal tubules are less than normal. The production of replacement dentin leads to obliteration of the pulp chamber and calcification of the root canals.
Post-Covid syndrome can manifest itself as:
- general physical weakness, dizziness,
- prolonged low-grade fever, fluctuations in body temperature,
- heaviness in the chest,
- difficulty breathing,
- vascular symptoms (vasculitis, microcirculatory disorders),
- heartbeat, fluctuations in blood pressure and pulse,
- pain in joints, muscles,
- headaches,
- loss and distortion of smell and taste,
- digestive disorders, including diarrhea,
- sleep disorder,
- anxiety, panic attacks,
- cognitive decline, memory loss, brain fog
- hair loss, tooth loss, cystic formations of the jaws,
- thermoregulation disorder, etc.
The severity and duration of manifestations depends on the course of the infectious process. Treatment consists of symptomatic therapy and includes medications, exercise therapy and physiotherapy.
Causes
The main cause of post-Covid syndrome is a new coronavirus infection. The duration of the disease depends on the severity and can be 2-3 weeks for mild cases, and 4-6 weeks or more for moderate and severe cases.
With this disease, the patient may retain certain symptoms, which researchers associate with:
- persistent inflammatory process,
- possible long-term presence of the SARS-CoV-2 virus in the body,
- development of autonomic dysfunction,
- chronic stress caused by lifestyle changes (introduction of quarantine measures).
Treatment
Currently, there are no treatment methods that can completely get rid of any myotonic syndrome . Treatment is symptomatic. If myotonic attacks become intense, it becomes necessary to use medications to reduce symptoms. The most well-known drug is mexilitene, as well as drugs such as guanine, procainamide, tegretol, phenytoin. But all these drugs have a lot of side effects and therefore their long-term use is not advisable. It is most optimal when the patient knows the factors that provoke myotonic attacks and tries to avoid provoking situations whenever possible, and after the attacks allows the muscles to recover through rest.
Pathogenesis
The mechanism of occurrence of post-Covid syndrome is poorly understood and is presumably related to the morphological structure and properties of the virus and its effect on human tissues and organs.
One of the reasons is the tropism of the virus to the vascular endothelium of nervous, renal, and pulmonary tissue and the ability to cause chronic vasculitis in it with the development of thromboembolism, tissue hypoxia and organ ischemia.
The development of a pathological immune response cannot be ruled out, leading to the development of chronic autoimmune inflammation that affects human organs and systems.
Separately, one should take into account the influence of the SARS-CoV2 virus on the development of autonomic dysfunction, which is clinically manifested in dysfunction of the cardiovascular system, gastrointestinal tract, respiratory and nervous systems.
Hereditary enamel dysplasia
Amelogenesis imperfecta is understood as a genetically determined pathology in which the structure of the enamel is disrupted. The disease develops at the stage of laying ectodermal sheets, from which the bone structure, blood vessels and a number of internal organs are subsequently formed.
Amelogenesis imperfecta is more often diagnosed in women. This fact is due to the fact that the abnormal development of teeth of this type provokes damage to other structures, resulting in the death of the male fetus.
The anomaly first appears in children when the growth of baby teeth begins. The latter have an uncharacteristic structure with thinned enamel, which acquires a yellow or brown tint.
Provoking factors
Amelogenesis imperfecta develops through the transmission of mutated material (the amelogenin gene) via the X chromosome or autosome. These changes lead to the formation of thin crystals, from which tooth enamel is subsequently formed.
Mutations of other genes also influence the development of enamel dysplasia. In particular, due to the imperfection of MMP-20, the fermentation of calcium-dependent proteinase is disrupted. The latter is responsible for the formation of the enamel matrix.
Type of enamel dysplasia
In medical practice, it is customary to distinguish four types of amelogenesis:
- Hypoplastic . It occurs at the stage of formation of dental tissues. The hypoplastic type is characterized by dysfunction of ameloblasts.
- Hypomaturation . Occurs against the background of disruption of the enamel formation process. In this case, the normal thickness of tooth enamel is maintained. However, it lacks minerals.
- Hypocalcification . It develops due to a failure in the mineralization process of tooth enamel. This type is characterized by active growth of crystallites. As a result, this anomaly leads to a decrease in the volume of minerals in the enamel.
- Hypomaturation with hypoplasia . The intensity of the symptoms is determined by the summation of the signs of both pathologies.
Features of symptoms
Regardless of the type of disease, it is accompanied by the following manifestations:
- thinning of enamel;
- darkening of its surface;
- the formation of depressions on the teeth located near the cheeks;
- partial or complete erasure of enamel and dentin.
The hypoplastic type of the disease is characterized by:
- darkening of the enamel surface;
- delay in teething;
- decreased bite height;
- displacement of the head related to the temporomandibular joint.
With the hypomaturation type, the following are observed:
- low X-ray density of enamel caused by its low mineral content;
- The upper parts of the tooth are covered with small dots or stripes.
The hypocalcification type occurs accompanied by the following phenomena:
- the enamel becomes soft, which leads to its peeling;
- the appearance of frequent chips on the surface of the teeth;
- the shine of teeth is lost.
Due to the fact that amelogenesis leads to thinning of the enamel, due to its gradual destruction, teeth acquire various abnormal shapes.
What does modern medicine offer?
Treatment of amelogenesis imperfecta takes about one year. For this disease the following are indicated:
- applications with preparations containing fluoride and calcium, this procedure is repeated every three months;
- use of casein phosphoprotein and amorphous calcium phosphate;
- restoration and prosthetics of teeth in case of diagnosis of significant damage.
Timely treatment, during which problem teeth are covered with crowns, can also achieve a positive effect.
Symptoms
There is no single picture and clinical manifestations of post-Covid syndrome. Some patients experience the same symptoms as during the illness, while others develop new symptoms. The most common symptoms include:
- constant fatigue,
- disturbance of circadian rhythms (sleep and wakefulness),
- periodic low-grade fever or hypothermia,
- pain, congestion, heaviness in the chest,
- cough and shortness of breath,
- irritability, tearfulness, anxiety,
- headache,
- heart pain, heart rhythm disturbances,
- hyper- or hypotension,
- hair loss,
- impaired skin sensitivity,
- hearing and smell impairment,
- indigestion,
- loss of concentration and memory.
Symptoms
Stanton-Capdepont syndrome does not prevent teeth from erupting on time. Early or late appearance is rare. A characteristic symptom of the disease is the color of the enamel - from yellow to gray and brown. It occurs due to the filling of the dentinal tubules with blood. When chipped, the enamel exposes the dentin surface.
An increase in water content and a decrease in mineral components destroys the dentin structure, which affects increased tooth wear. A low bite reduces the lower third of the face. Due to chipped enamel, the edges of the teeth become sharp, resulting in painful damage to the mucous membrane. What is glass ionomer cement used for? More on this later.
Diagnostics
A therapist, cardiologist or neurologist can diagnose post-Covid syndrome, depending on the reason for treatment and symptoms of manifestation. The patient may be prescribed the following examinations:
- Laboratory diagnostics.
Includes quantitative and qualitative analysis of immunoglobulins of classes M and G to SARS-CoV-2. It is possible to conduct clinical and biochemical blood tests, as well as coagulation tests.
- Instrumental diagnostics.
Studies prescribed for these disorders may include: ECG, echocardiogram, 24-hour Holter heart rate monitoring, laser Doppler flowmetry, active orthostatic test, olfactory test, electroencephalogram. For other complaints, depending on the symptoms, an MRI or ultrasound of the abdominal organs and pelvis may be prescribed. Ultrasound scanning of veins or arteries.
- Psychodiagnostics
. Patients with anxiety and depressive symptoms and cognitive impairment require consultation with a clinical psychologist or psychotherapist.
Cardiorenal syndrome: etiology, diagnosis, therapy.
According to studies conducted in Europe and the USA, the prevalence of CHF in the population varies from 0.4 to 2% and increases significantly in people over 60 years of age, reaching 10% [1].
As for the Russian Federation, according to I.V. Fomin et al. in the European part of the country, CHF affects 8.9% of the total population and 54% in people over 80 years of age [2].
Thus, the prognosis for patients with CHF is extremely unfavorable; mortality among this group of patients is 4-8 times higher than in the general population of the same age [3, 4, 5].
Further study of the problem of CHF showed that a simultaneous deterioration in kidney function aggravates the course of the disease and significantly increases the risk of death. This risk becomes more pronounced when serum creatinine levels increase above 1.3 mg/dL and when glomerular filtration rate (GFR) decreases below 60 ml/min [6, 7].
Thus, the kidneys are considered not only as an organ that contributes to the development of edema syndrome, but also to the progression of CHF. The kidneys retain Na+ and water, increase preload, and activate the renin-angiotensin-aldosterone system (RAAS), which leads to the development of myocardial hypertrophy with subsequent dilatation of the left ventricle. The coexistence of two pathological processes in one patient served as the basis for combining them into a single term, called cardiorenal syndrome (CRS) [8].
Currently, CRS is a pathological interdependent condition involving the heart and kidneys, developing as a result of acute or chronic dysfunction of one of the organs followed by acute or chronic dysfunction of the other.
Currently, there is a classification of cattle, consisting of 5 types, depending on the pathophysiological and time frame for the development of cardiac and renal failure. CRS can be represented as a condition in which, as a result of acute or chronic dysfunction of one organ, acute or chronic dysfunction of another organ occurs [9].
Type 1 RRS is a sudden, acute deterioration in cardiac function that results in acute kidney injury (AKI). Type 1 CRS occurs in acute coronary syndrome in 9-19% of cases [10]. Acute decompensation of CHF is complicated by AKI in 24-45% of cases [11, 12]. Clinical and laboratory manifestations of AKI usually develop in the first 4 days (50% of cases) or within 7 days (70-90% of cases) [13].
Type 2 KRS is characterized by the patient having CHF, which over time leads to the formation and progression of chronic kidney disease (CKD). The incidence of CKD among patients with CHF can be 45-63.6% and is a poor predictor of cardiovascular death [14, 15].
Type 3 RRS, or acute renocardial syndrome, involves primary and acute renal dysfunction resulting from acute glomerulonephritis, pyelonephritis, or acute tubular necrosis. In turn, AKI causes heart failure, arrhythmia and myocardial ischemia. AKI is most common in patients in intensive care units (35%) [16].
Cattle type 4 is characterized by CKD, which leads to CHF. The prevalence of CKD around the world has been increasing recently and reaches 10-15%. Currently, the main causes of CKD are diabetes mellitus, arterial hypertension, atherosclerosis and obesity, that is, diseases that are most common in developed countries. The risk of death in patients with CKD from cardiovascular diseases increases 10-20 times compared with patients without CKD [17, 18].
Type 5 RRS is a combination of cardiac and renal pathology resulting from acute injury, in which dysfunction of one organ affects the function of another organ. Most often, type 5 KRS develops in septic patients. Sepsis is the most common condition that causes dysfunction of the myocardium and kidneys [19, 20].
Cattle was presented by A. Guyton (1990) in the form of a hemodynamic model. According to this model, the kidneys control extrarenal fluid by regulating the processes of Na+ excretion and reabsorption, and the heart controls systemic hemodynamics [21]. The central place is given to the RAAS, natriuretic peptide (NUP) and the kallekrein-kinin system. As a result of damage to one of the organs, the RAAS and sympathetic nervous system are activated, endothelial dysfunction and chronic inflammation develop. The result is a vicious circle in which the combination of cardiac and renal dysfunction leads to an accelerated decline in the functional capacity of each organ.
The pathogenesis of BRS depends on the type of syndrome. In cattle type 1, AKI occurs as a result of decreased cardiac output, which leads to impaired renal perfusion. Resistance to diuretic therapy often develops. Attempts to relieve edema or AKI by increasing the dose or using a combination of diuretics may be an additional factor in the progression of AKI.
The pathogenesis of type 2 RRS is based on prolonged renal hypoperfusion and activation of vasoconstrictors such as adrenaline, angiotensin and endothelin.
In type 3 cattle, as a result of AKI, hyperhydration occurs, which can lead to pulmonary edema and hyperkalemia, which contributes to arrhythmia and cardiac arrest.
Early diagnosis of cattle allows you to start treatment in a timely manner, prevent the development of complications and reduce mortality. Currently, great importance is attached to the determination of biomarkers of the disease. A biomarker should be detected in the early stages of the disease, indicate the time and severity of damage, have high sensitivity and specificity, and allow one to predict the course of the disease.
A biomarker that is detected in the early stages of AKI is neutrophil gelatinase-associated lipocalin (NGAL), the appearance of which precedes the increase in creatinine by 48-72 hours [22, 23].
In accordance with data presented by RG VandeVoorle (2006), cystatin C correlates with the duration and severity of AKI, the need for renal replacement therapy (RRT) and hospital mortality [24].
Kidney injury molecule (KIM-1) is a protein that is found in the urine after ischemic or toxic damage to the proximal tubules. KIM-1 is an important and highly sensitive biomarker of the early stages of AKI [25].
In addition to the above biomarkers, in scientific publications you can find references to interleukin-18 and the lysosomal enzyme N-acetyl-β-d-glucosaminidase, which allow diagnosing AKI [26].
Regarding the diagnosis of acute myocardial injury, it is necessary to note NUP, which is detected in acute heart failure or decompensated CHF [13, 27].
A generally accepted marker of myocardial necrosis is troponin. Elevated troponin levels are associated with increased mortality in CKD and have prognostic significance for type 4 cattle [20].
Markers of CKD progression currently include microalbuminuria, proteinuria, C-reactive protein levels, and decreased GFR [28].
Serum creatinine is a simple but important indicator for determining the survival of patients with CHF. According to the results presented by GI Smith et al. (2003) an increase in serum creatinine concentration during hospitalization by 0.2 mg/dL or more increases the risk of death over the next 6 months by 67% and the likelihood of readmission by 33% [12].
Cattle therapy includes the following measures: prescription of diuretics, ACE inhibitors, nitrates and cardiac glycosides.
It should be noted that long-term therapy of cattle, in particular the use of diuretics, can lead to a decrease in their effectiveness and the formation of a condition refractory to drug therapy [29, 30].
One of the effective methods of treating severe cattle refractory to drug therapy is the use of RRT methods. RRT methods, in particular ultrafiltration (UF), hemodialysis (HD), hemofiltration (HF), hemodiafiltration (HDF), are widely used in nephrological practice and in the treatment of critical conditions. One of the components of HD, GF, HDF is UV, that is, the removal of liquid. A number of studies have noted the effectiveness of UV as a method of choice in the treatment of severe cattle [31, 32, 33, 34, 35].
The positive effect of UV as a component of RRT is due to the elimination of hyperhydration, reducing the load on the heart due to a decrease in venous return, which ultimately affects the improvement of the contractile function of the heart. Good tolerability of sessions is due to maintaining the stability of the electrolyte composition and plasma osmolarity, which ensures an adequate influx of interstitial fluid into the vascular bed. RRT methods allow you to immediately begin to extract fluid from the body, remove it at a given speed and in the required volume [36, 37].
An important point is the ability of RRT to reduce the concentration of uric acid (UA) in the blood serum. In the observed patients, as noted above, an increased level of sUA was determined. The data presented by S. R. Gilyarevsky show a connection between the level of uric acid and the risk of worsening the course of cattle disease and, as a consequence, a likely unfavorable outcome of the underlying disease [38]. On the other hand, an increase in UA levels can be provoked by the use of high doses of furosemide. This can ultimately create the preconditions for the formation of urate block, which in turn can worsen the function of already compromised kidneys. RRT eliminates this component, which can aggravate the course of cattle. Normalizing the level of sUA to a certain extent helps to normalize the rate of diuresis.
The use of one of the RRT techniques - UV in cattle therapy is described by MR Costanzo et al. [39]. The paper presents the results of a multicenter randomized study comparing the effectiveness of UV with intravenous diuretics in patients with acute decompensated CHF. The UV group achieved greater fluid removal, resulting in greater weight loss compared to the diuretic-only group. After 90 days, in the group where UV was performed, readmission to hospital was observed only in 18% of cases versus 32% in the group where only diuretics were used [34].
In conclusion, a “hybrid” technique called slow low-efficiency daily dialysis (SLEDD) has emerged in recent years. This method allows you to prevent rapid fluctuations in the concentration of substances and/or a decrease in intravascular volume due to a longer session time, exceeding 4 hours. The fact is that patients with CHF are overloaded with fluid, which accumulates in the third space (ascites, hydropericardium, hydrothorax). However, in reality, these patients experience an imbalance in the distribution of fluid in the body, in which hypovolemia is quite often observed in the vascular bed. When performing UV, an imbalance between the distribution of fluid in the body can lead to hypotension. The use of the SLEDD technique allows maintaining hemodynamic stability due to the low UV speed. In addition, during SLEDD sessions, adequate clearance of low molecular weight water-soluble substances is maintained and there are no severe electrolyte disturbances [40, 41].
Cattle has been encountered quite frequently in recent years. This is due to the progressive increase in cardiovascular pathology, diabetes mellitus, obesity and increasing life expectancy of the population. The development of CRS in CHF complicates treatment and significantly worsens the prognosis of the disease. It is likely that deterioration of renal function is a key factor leading to decompensation of CHF. At a certain stage of CHF progression, the kidneys become an even more important target organ than the heart. Thus, the main direction of the treatment strategy for CHF and cattle is the creation of nephroprotective therapy, which will slow down the progression of the pathology, increase the duration and quality of life of patients with CHF.
Bibliography
- Reznik E. V., Strozhakov G. I., Gendlin G. E. The heart is sick and the kidneys are suffering: cardiorenal syndrome in patients with chronic heart failure. General Medicine 2009; 1:27–35.
- Fomin I.V., Belenkov Yu.N., Mareev V.Yu. et al. Prevalence of CHF in the European part of the Russian Federation - data from EPOCHA-CHF. Heart disadvantage 2006; 1 (35): 4–8.
- Ageev F. E., Skvortsov A. A., Mareev V. Yu. et al. Heart failure against the background of coronary heart disease: some issues of epidemiology, pathogenesis and treatment. Rus. honey. zhurn 2000; 15/16: 622–626.
- Heywood JT The cardiorenal syndrome: Lessons from the ADHERE data base and treatment options. Heart Fail. Rew 2004; 9: 195–201.
- Mc Alister F. A., Ezekowitz J., Tonelli M. et al. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004; 109:1004–1009.
- Dries DL, Exner DV, Domanski MJ et al. The prognostic implications of renal insufficiency in a symptomatic and symptomatic patients with left ventricular systolic dysfunction. J. Am. Coll. Cardiol 2000; 35:681–689.
- Mahan NG, Blackstone EH, Francis GS et al. The prognostic value of estimated creatinine clearance alongside functional capacity in patients with chronic congestive heart failure. J. Am. Coll. Cardiol 2002; 40: 1106–1113.
- Ronco C. Cardiorenal and renocardial syndromes: clinical discorders in search of a systematic definition. Int. J. Artif. Organs 2008; 31:1–2.
- Ronco C., Haapio M., House A.A. et al. Cardiorenal syndrome. J. Am. Coll 2008; 52:1527–1539.
- Latchamsetty R., Fang J., Kline-Rogers E. et al. Prognostic Value of Treatment and Sustained Increase in In-Hospital Creatinine on Outcomes of Patients Admitted With Acute Coronary Syndrome. Am. J Cardiol 2007; 99(7):939–942.
- Cowie M.R., Komajda M., Murray-Thomas T. et al. Prevadence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH). EH J 2006; 27: 1216–1222.
- Smith G.L., Vaccarino V., Kasiborod M. et al. Worsening renal function: What is a clinically meaningful change in creatinine during hospitalization with heart failure? J.Card. Fail 2003; 9:13–25.
- Ronco C., Bellomo R., McCullough PA Cardiorenal Syndrome in Critical Care. Contr. Nephrol 2010; Based Karger 2010; 165
- Ahmed A., Rich MW, Sanders PW et al. Chronical Kidney Disease Associated Mortality in Diagnostic Versus Systolic Heart Failure: A Propensity Matched Study. Am. J Cardiol 2007; 99: 393–398.
- Campbell R.C., Sui S., Flippatos G. et al. Association of chronic kidney disease with outcome in chronic heart failure: a propensity-matched study. Nephrol. Dial. Transplant 2009; 24: 186–193.
- Roghi A., Savonitto S., Cavallini C. et al. Impact of acute renal failure following percutaneous coronary intervention on long-term mortality. J. Cardiovasc. Med 2008; 9: 375–381.
- Coresh IJ , Stevens L. , Levery A. Chronic kidney disease is common: What do we do next? Nephrol. Dial. Transplant 2008; 23(8):1122–1125.
- McClellan W. The epidemic of renal disease – what drives it and what can be done? Nephrol. Dial. Transplant 2006; 21(6):1461–1464.
- Bagshaw S. M., Lapinsky S., Dial S. et al. Acute kidney injure in septic shock: clinical outcome and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 2009; 35:871–881.
- Ammann P., Maggiorini M., Bertel O. et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J. Am. Coll. Cardiol 2003; 41: 2004–2009.
- Bongartz LG, Cramer MJ, Doevendans PA et al. The severe cardiorenal syndrome. Eur. Heart. J 2005; 26: 11–17.
- Han WK , Bonvente JV Biologic markers for the early direction of acute kidney injury. Curr. Opin. Crit. Care 2004: 10: 476–482.
- Ronco C. NGAL: an emerging biomarker of acute kidney injury. Int. J. Artif. Organs 2008; 11: 199–200.
- VandeVoorde RG, Katlman TI, Ma Q. et al. Serum NGAL and cystain C as predictive biomarkers of acute kidney injury. J. Am. Soc. Nephrol 2006; 17:404A.
- Parkh CR, Devarajan P. New biomarkers of acute kidney injury. Crit. Care Med 2008; 36(Suppl. 4): 159–165.
- Kobalava Zh. D., Efremovtseva M. A., Villevalde S. V. Cardiorenal syndromes. Wedge. Nephrol 2011; 6:9–15.
- Austin W. J., Bhalla V., Hernandez-Arce I. et al. Crelation and prognostic utility of B-type natriuretic peptide and its aminoterminal fragment in patients with chronic kidney disease. Am. J. Clin. Pathol 2006; 126:506–512.
- Chadban SJ, Briganti EM, Kerr PG et al. Prevalence of kidney damage in Australian adults: The usDiab kidney study. J. Am. Soc. Nephrol 2005; 14 (Suppl.): 131–138.
- Geisbery C., Butlet J. Addressing the challenges of cardiorenal syndrome. Clew. Clin. J Med 2006; 73:485–491.
- Liang KV, Williams AW, Greene EL et al. Acute decompensated heart failure and the cardiorenal syndrome. Crit. Care Med 2008; 31(1Suppl): 75–88.
- Jaski BE, Miller D. Ultrafiltration in decompensated heart failure. Curr. Heart Fail Rep 2005; 2: 148–158.
- Wertman BM, Gura V. Ultrafiltration for the Management of Acute Decompensated Heart Failure. J.Card. Fail 2008; 14: 754–759.
- Marenzi G., Lauri G., Grazi E. et al. Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive Heart Failure. J. Am. Col. Cardiol 2001; 38(4):963–968.
- Costanzo MR, Saltzberg M, O'Sullivan J et al. Early ultrafiltration in patients with decompensated Heart Failure and diuretic resistanse. J. Am. Col. Cardiol 2005; 46(11):2047–2051.
- Udani SM, Murray PT The use of renal replacement therapy in acute decompensated heart failure. Semin. Dial 2009; 22 (2): 173–179.
- Violi F., Nacca R.G., Lengo G. et al. Long-term sustained low efficiency dialysis in eight patients with class IV NYHA heart failure resistant to high-dose diuretic treatment. G. Ital. Nefrol 2009; 26 (Suppl. 46): 50–52.
- Hillege HL , Nitsch D. , Pfeffer MA Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113:671–678.
- Gilyarevsky S. R. Hyperuricemia and chronic heart failure: is there a cause-and-effect relationship? Wedge. Nephrol 2010; 5: 18–22.
- Costanzo MR, Guglin ME, Saltzberg MT et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J. Am. Coll. Cardiol 2007; 49: 675 – 683 (Erratum in 2007; 49: 1136).
- Unarokov Z. M., Mukhoedova T. V. Renal replacement therapy in the treatment of diuretic-refractory edema in patients with cardiorenal syndrome. Sat. mater. "Act. aspects of extracorporeal blood purification in intensive care." M 2010: 27.
- Bockeria L. A., Yarustovsky M. B. Guidelines for extracorporeal blood purification in intensive care. M.: NTsSSKh them. A. N. Bakuleva RAMS, 2009 – 486 p.
Treatment of post-Covid syndrome
If the attending physician suspects that the patient has post-Covid syndrome, a consultation at the center is recommended. If tests for the SARS-CoV2 virus are negative, the attending physician sends patients or a package of documents to the selection committee at the specified center. The selection committee coordinates an outpatient consultation at the Center and prepares primary related documentation. If indicated, after an outpatient consultation, the patient may be recommended to be hospitalized for one day to conduct further studies and receive therapy. The attending physician of the Center invites patients for further treatment and rehabilitation. If necessary, dispensary observation is established, health education is carried out, increasing patients' awareness of this disease and increasing motivation for treatment and rehabilitation.
Treatment includes symptomatic pharmacotherapy, physical rehabilitation and exercise therapy, and physiotherapy.
How can prosthetics help?
Prosthetics is the optimal method of restoring the function and aesthetics of teeth. Solid crowns are made for the lateral teeth, while the front ones are covered with metal-ceramic crowns. Prosthetics can also be carried out by veneering, lamination of the anterior group of teeth and cheek surfaces using ceramic onlays. If the root of a tooth is fractured or if endodontic therapy is poorly effective, the tooth is removed. To replace defects in the dentition, removable dentures are often used.