What is arsenic and where is it used?
Arsenic, also known as Arsenicum in Latin, is an element of the periodic table, a semimetal. Despite the fact that it is a strong poison for the human body, arsenic also has beneficial properties, which makes it indispensable in various fields:
- agriculture (pesticides),
- deratization and disinfestation (the element is included in many pest control products),
- production of semiconductors and electronics,
- glass industry,
- metallurgy (to impart strength to alloys and increase their anti-corrosion resistance),
- woodworking (for impregnation of wooden products),
- leather and fur industry (as an antiseptic for products),
- medicine (in particular dentistry).
In nature, Arsenicum is present in certain quantities in ore deposits, mountain and earth layers, from where it can be washed out with rain into nearby bodies of water. And when using such water, poisoning is very likely. But this is not the only situation in which arsenic can cause poisoning.
How was Arsenic discovered?
The chemical element arsenic has been known to people since ancient times. As early as the third millennium BC, people encountered this element when they mined copper, where it is a by-product. Later, it began to be used as an impurity in an alloy, which in its properties resembles bronze. Today it is believed that the first person to isolate arsenic was the German Catholic Albertus Magnus. This event supposedly took place in 1250. He restored this element with coal. Also in 1649, Johann Schröder published 2 different methods for obtaining pure arsenic. Subsequently, its study by scientists made it possible to learn many of its properties. It was quite popular and found application in many areas. These areas include medicine (cosmetics and medicine), military affairs (chemical weapons) and foundry production.
Read: Germanium as a chemical element on the periodic table
Ways and methods of poisoning
There are only 3 routes of intoxication: inhalation, ingestion and skin contact. This can happen in different ways:
- in cases of murder or suicide attempts, industrial accidents,
- when living in arsenic-contaminated areas,
- inhalation of poisoned air,
- after eating seafood from contaminated waters,
- from the use of pesticides, preservatives (food), herbicides and products against insects, rodents and fungi,
- after drinking raw water,
- in direct contact with semi-metal in production,
- violation of safety regulations when working with arsenic.
In other words, you can encounter arsenic almost everywhere. But whether a person becomes poisoned or not will depend on the concentration of the poison that enters the body.
PHYSICAL PROPERTIES
| Mineral color | pewter-white, fading to dark gray or black on the surface |
| Stroke color | grey |
| Transparency | opaque |
| Shine | semi-metallic, dull |
| Cleavage | perfect by {0001} and less perfect by {0112} |
| Hardness (Mohs scale) | 3,5 |
| Kink | uneven |
| Strength | fragile |
| Density (measured) | 5.63 – 5.78 g/cm3 |
| Radioactivity (GRapi) | 0 |
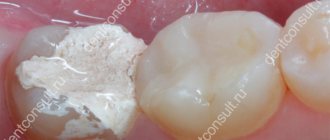
Arsenic in dentistry - is it dangerous?
Not so long ago, arsenic paste was used to successfully kill the dental nerve by placing it in the cavity of an exposed diseased tooth. It was assumed that over the next 2 days the poison would reduce sensitivity to zero, the nerve would be removed, and the tooth would be healed.
It would seem that everything is very simple. But there are some nuances here: you can carry arsenic in your mouth for no longer than 2 days. After this time, a visit to the dentist must be made again to remove the paste. Because of this, the question often arises: is it possible to get poisoned by carrying arsenic paste in your mouth?
Oddly enough, many people, afraid to feel pain again, walk with arsenic in their mouth for more days than they should, in order to be sure to “finish off” the nerve. The result here can be one: the poison begins to have a destructive effect on the tooth and soft tissues, which leads to inflammation and rapid destruction of the tooth itself.
Contrary to the existing myth that a filling with arsenic can poison the body, I would like to say that this is not at all the dose of poison that could really lead to intoxication. With arsenic, dental treatment will not lead to poisoning, even if:
Read also: Watermelon poisoning in humans
- a dental filling containing arsenic fell out and was accidentally swallowed,
- the patient is a child,
- There was an overexposure of the arsenic filling in the mouth.
The minimum amount of arsenic in the composition of the medicinal paste is unable to poison the entire body. But since it can harm the tooth, this substance is rarely used in dentistry. Basically, unless the patient is allergic to modern local anesthetics.
And although arsenic poisoning during dental treatment is impossible, dentists themselves increasingly refuse to use the outdated method of killing the nerve in order to preserve the tooth and its soft tissues. This has been replaced by strong anesthetics that can reduce discomfort to zero, which allows you to painlessly treat the tooth and preserve it for a long time.
MORPHOLOGY
Arsenic is usually observed in the form of crusts with a sintered kidney-shaped surface, stalactites, shell-like formations, which reveal a crystalline-granular structure when fractured. Native arsenic is quite easily recognized by the shape of the deposits, blackened surface, significant specific gravity, strong metallic luster in a fresh fracture and perfect cleavage. Under the blowpipe it evaporates without melting (at a temperature of about 360°), emitting a characteristic garlic odor and forming a white As2O3 coating on the coal. It turns into a liquid state only at increased external pressure. In a closed tube it forms a mirror of arsenic. When struck sharply with a hammer, it emits a garlicky smell.
Impact on the body
So, it is impossible to be poisoned by this semi-metal during dental treatment. However, if the poison enters the body in other ways and in large quantities, serious harm is caused to health.
A lethal dose of arsenic for an adult can be 0.1 - 0.2 g of poison. Sometimes a negligible dose of 0.05 g is enough for a child.
Under the influence of arsenic (after its rapid absorption into the blood), all organs and their systems are affected. The liver, heart, lungs and kidneys are especially affected, and this happens in just a day. After 2 weeks, the substance is found in bones, nails, hair and skin. Wherein:
- biochemical processes are disrupted,
- the nervous system is affected,
- oxygen exchange in cells is disrupted,
- the substance quickly binds to hemoglobin, etc.
Removal of poison from the body during arsenic poisoning occurs with feces and urine, and with urine much faster. The remnants of the received dose are stored in tissues and organs, continuing to have a detrimental effect.
Safety regulations
When buying rice, you should remember that it may contain traces of a toxic substance. Therefore, purchased rice in bulk should be thoroughly washed and dried. Another way to protect your body from the effects of harmful impurities is to increase the water when cooking rice, in a ratio of 1:6. An effective, albeit unconventional, method is to cook the rice using a coffee maker. This idea came from American scientists. The device allows you to evaporate rice, thereby destroying toxic components.
To avoid possible arsenic poisoning, rice should be cooked in plenty of water.
Considering the harmfulness of arsenic present in rice, it is worth reducing its daily consumption and partially replacing it with other products. Nutrition experts recommend amaranth, barley, and whole wheat pasta. Obviously, you should not obsessively get rid of rice reserves, since eating the product in small quantities will not harm the body at all. You just have to observe moderation and maintain common sense. Take care of yourself and always be healthy!
Symptoms
Signs of arsenic poisoning in acute form are quite characteristic. This specificity helps to identify the problem in time:
- temperature increase,
- thirst, dehydration,
- vomiting with nausea,
- sharp pain of varying intensity in the stomach,
- decreased diuresis,
- weakness,
- loose stools that look like rice water,
- numbness of hands and feet,
- metallic taste in the mouth,
- convulsions,
- partial loss of vision,
- the characteristic smell of garlic from the mouth of a poisoned person,
- heart failure, tachycardia, decreased blood pressure.
Read also: Meat poisoning in humans
These symptoms of arsenic poisoning indicate an acute form. But with constant contact with this poison (for example, when working in production where Arsenicum is used), the intoxication takes on a chronic form.
Chronic arsenic poisoning will not be immediately noticeable, but it will eventually result in very serious problems due to the growing amount of poison that gradually accumulates in the body:
- the upper layers of the skin grow and become scab-like (hyperkeratosis),
- whitish stripes appear on the nail plates of the hands and feet,
- the skin peels and flakes off even in those parts of the body that have always been protected from contact with poison,
- red spots appear on the head, chest, armpits and scrotum,
- encephalopathy and neuropathy develop,
- convulsions,
- destruction of red blood cells - hemolysis,
- toxic hepatitis,
- burns of the larynx, ulcers of the esophagus, bleeding in the gastrointestinal tract,
- renal failure with blood in urine darkened due to hemolysis,
- coma.
If acute arsenic poisoning is accompanied by symptoms that develop half an hour after taking a dose of poison, then in the chronic form, signs of intoxication can be noticed only after 0.5 - 2 months.
First aid for arsenic poisoning
First aid for arsenic poisoning is carried out as standard.
- Call an ambulance.
- If there is no consciousness, place the injured person on his side.
- If there are no signs of life, cardiopulmonary resuscitation is performed.
- drinking small amounts frequently
Get rid of any remaining toxin. When the person is conscious, it is necessary to lavage the stomach with plenty of water. You can add 2 teaspoons of salt per liter of water. This poison is easily removed from the skin with water, soap and a washcloth.
- Combating intoxication and dehydration includes drinking small amounts frequently.
- You can take activated carbon. But you shouldn’t expect a strong effect, since coal weakly binds arsenic. It is better not to take laxatives.
For mild poisoning, hospitalization may not be required. Victims in serious condition, as well as those of moderate severity, should be sent to a hospital.
How to help the victim
Although mild poisoning does not require hospital treatment, only a doctor can determine the severity of the problem. Therefore, first of all, you need to call an ambulance, and during its journey, independently help the victim:
- provide fresh air,
- give 1 glass or 1 liter of acidified water to drink (with 1 liter of vinegar or 3 g of citric acid) to cleanse the stomach,
- if there is hydrogen sulfide water in the house, give it a solution (100 ml) to neutralize the poison and transform it into a safe substance - arsenic sulfide,
- give sorbents (any except activated carbon, which is useless in this situation),
- in case of dehydration, unsolder the victim little by little, but often,
- If a toxic substance gets on your skin, wash it off with soapy water.
It is unlikely that in any home there will be drugs for every occasion, much less antidotes against arsenic. Therefore, all other actions will be carried out by doctors.
Historical information.
Also on the topic:
ARSENIC AND HUMAN HEALTH
Arsenic belongs to the five “alchemical” elements discovered in the Middle Ages (surprisingly, four of them - As, Sb, Bi and P - are in the same group of the periodic table - the fifth). At the same time, arsenic compounds have been known since ancient times; they were used to produce paints and medicines. Particularly interesting is the use of arsenic in metallurgy.
Several thousand years ago, the Stone Age gave way to the Bronze Age. Bronze is an alloy of copper and tin. Historians believe that the first bronze was cast in the Tigris-Euphrates valley, somewhere between the 30th and 25th centuries. BC. In some regions, bronze with especially valuable properties was smelted - it was better cast and easier to forge. As modern scientists have found, it was a copper alloy containing from 1 to 7% arsenic and no more than 3% tin. Probably, at first, during its smelting, the rich copper ore malachite was confused with the weathering products of some also green sulfide copper-arsenic minerals. Having appreciated the remarkable properties of the alloy, the ancient craftsmen then specifically looked for arsenic minerals. For the search, we used the property of such minerals to give off a specific garlic odor when heated. However, over time, the smelting of arsenic bronze ceased. Most likely this happened due to frequent poisoning during the firing of arsenic-containing minerals.
Of course, arsenic was known in the distant past only in the form of its minerals. Thus, in Ancient China, the solid mineral realgar (sulfide with the composition As4S4, realgar in Arabic means “mine dust”) was used for stone carving, but when heated or exposed to light, it “deteriorated” as it turned into As2S3. In the 4th century. BC. Aristotle described this mineral under the name "sandarac". In the 1st century AD The Roman writer and scientist Pliny the Elder, and the Roman physician and botanist Dioscorides described the mineral orpiment (arsenic sulfide As2S3). Translated from Latin, the name of the mineral means “golden paint”: it was used as a yellow dye. In the 11th century alchemists distinguished three “varieties” of arsenic: the so-called white arsenic (As2O3 oxide), yellow arsenic (As2S3 sulfide) and red arsenic (As4S4 sulfide). White arsenic was obtained by sublimation of arsenic impurities during the roasting of copper ores containing this element. Condensing from the gas phase, arsenic oxide settled in the form of a white coating. White arsenic has been used since ancient times to kill pests, as well as...
In the 13th century Albert von Bolstedt (Albert the Great) obtained a metal-like substance by heating yellow arsenic with soap; This may have been the first example of arsenic in the form of a simple substance obtained artificially. But this substance violated the mystical “connection” of the seven known metals with the seven planets; This is probably why alchemists considered arsenic a “bastard metal.” At the same time, they discovered its property of giving copper a white color, which gave rise to calling it “a Venus (i.e. copper) bleaching agent.”
Arsenic was clearly identified as an individual substance in the mid-17th century, when the German pharmacist Johann Schroeder obtained it in a relatively pure form by reducing the oxide with charcoal. Later, the French chemist and physician Nicolas Lemery obtained arsenic by heating a mixture of its oxide with soap and potash. In the 18th century arsenic was already well known as an unusual "semi-metal". In 1775, the Swedish chemist K.V. Scheele obtained arsenic acid and gaseous arsenous hydrogen, and in 1789 A.L. Lavoisier finally recognized arsenic as an independent chemical element. In the 19th century Organic compounds containing arsenic were discovered.
Treatment in hospital
If the poisoning is determined to be severe, the patient will be transported to the department. There, under medical supervision, he will receive the necessary treatment:
Read also: Soda poisoning in humans
- Antidotes. The introduction of an antidote is a necessary step in the treatment of poisoning. In case of acute arsenic intoxication, Unithiol will play this role as the main antidote. It neutralizes the poison, turning it into a safe compound, and removes it from the body along with urine. It is administered intravenously (injection or dropper) or intramuscularly. In the chronic form, D-penicillamine is used as an antidote - one gram orally 4 times a day.
- Oxygen. Inhalations with it are indicated for arsenic vapor poisoning.
- Atropine + morphine. They are administered by injection if pain in the stomach persists.
- Hemodialysis, blood transfusion, increased diuresis. They are carried out selectively depending on how badly the kidneys are damaged.
- Novocaine + glucose in the presence of blood in the urine.
- Calcium chloride (or saline + glucose + adrenaline) to maintain blood pressure and maintain fluid volume in the body.
Treatment after severe poisoning can last up to 2 years. Therefore, after an inpatient course, it is necessary to adhere to rehabilitation standards: do not violate the drinking regime, take baths with alkalis, follow the prescribed diet, and continue vitamin therapy.
STRUCTURE
The crystal structure of arsenic is ditrigonal-scalenohedral symmetry. Trigonal syngony, c. With. L633L23PC. The crystals are extremely rare and have a rhombohedral or pseudocubic habit.
Several allotropic modifications of arsenic have been identified. Under normal conditions, metallic or gray arsenic (alpha arsenic) is stable. The crystal lattice of gray arsenic is rhombohedral, layered, with a period a = 4.123 A, angle a = 54° 10′. Density (at a temperature of 20° C) 5.72 g/cm3; temperature coefficient linear expansion 3.36 • 10 degrees; specific electrical resistance (temperature 0° C) 35 • 10-6 ohm • cm; NV = f 147; coefficient compressibility (at a temperature of 30° C) 4.5 x 10-6cm2/kg. The melting point of alpha-arsenic is 816 ° C at a pressure of 36 atmospheres.
Under atm. Arsenic sublimes under pressure at a temperature of 615° C without melting. Heat of sublimation 102 cal/g. Arsenic vapors are colorless, up to a temperature of 800°C they consist of As4 molecules, from 800 to 1700°C - from a mixture of As4 and As2, above a temperature of 1700°C - only from As2. With the rapid condensation of arsenic vapor on a surface cooled by liquid air, yellow arsenic is formed - transparent soft crystals of a cubic system with a density of 1.97 g/cm3. Other metastable modifications of arsenic are also known: beta-arsenic - amorphous glassy, gamma-arsenic - yellow-brown and delta-arsenic - brown amorphous with densities of 4.73, respectively; 4.97 and 5.10 g/cm3. Above a temperature of 270° C, these modifications turn into gray arsenic.
Consequences
Severe poisoning often affects the body quite seriously, leaving behind additional health problems. After a strong (or long-term) toxic effect of arsenic (even after high-quality treatment), the following may appear:
- polyneuritis,
- decreased immunity with subsequent manifestation of existing chronic diseases,
- failure of the liver, lungs, kidneys, heart,
- headaches after exposure to the central nervous system (mainly felt in the back of the head, forehead and temples).
The consequences of poisoning in children are especially severe: they experience disturbances in speech, hearing and coordination of movements.
Arsenic is a strong carcinogen that, after poisoning, can cause cancer of the prostate, lung, kidney, bladder or liver.
Sources of entry of a chemical element into the body
Like other chemical elements, the semimetal enters the body mainly through drinking water and food. In developed countries, the content of this element in water corresponds to the norm; in poor countries, there is a problem of insufficient control of the composition of water, as a result of which local residents en masse become victims of the consequences of poisoning.
The foods richest in arsenic are grape wine and juices, fish and seafood; there is a lot of it in rice, grains and bread, lentils, as well as some vegetables (primarily carrots), berries, and plants.
Thus, the medicinal houseplant money tree or crassula contains this chemical element. Read more by following the link.
Some of the compounds enter the body with medications, fruits and vegetables containing herbicides and pesticides.
Prevention
Since arsenic is a poison that can cause not only disability, but also death, compliance with preventive measures is extremely important.
To protect yourself and others from the harmful effects of arsenic, it is enough to follow simple rules:
- in production, observe all necessary safety measures (protective uniform, neatness, etc.),
- do not shy away from undergoing medical examinations,
- do not drink raw water,
- do not buy products secondhand from strangers,
- Do not store substances containing arsenic in the house.
But if, nevertheless, it was not possible to avoid poisoning for some reason, the main thing is to remember the need to immediately call a doctor. You will not be able to determine the severity of intoxication on your own or completely remove the poison from the body.
ORIGIN
Arsenic occurs in hydrothermal deposits as metacolloidal formations in voids, apparently formed during the last moments of hydrothermal activity. In association with it, arsenic, antimonous, and, less commonly, sulfur compounds of nickel, cobalt, silver, lead, etc., of various compositions, as well as non-metallic minerals, can be found.
In the literature there are indications of the secondary origin of arsenic in weathering zones of arsenic ore deposits, which, generally speaking, is unlikely, given that under these conditions it is very unstable and, quickly oxidizing, decomposes completely. The black crusts consist of a fine mixture of arsenic and arsenolite (As2O3). Eventually pure arsenolite is formed.
In the earth's crust, the concentration of arsenic is low and amounts to 1.5 ppm. It is found in soil and minerals and can be released into the air, water and soil through wind and water erosion. In addition, the element enters the atmosphere from other sources. As a result of volcanic eruptions, about 3 thousand tons of arsenic are released into the air per year, microorganisms produce 20 thousand tons of volatile methylarsine per year, and as a result of the combustion of fossil fuels, 80 thousand tons are released over the same period.
On the territory of the USSR, native arsenic was found in several deposits. Of these, we note the Sadon hydrothermal lead-zinc deposit, where it was repeatedly observed in the form of kidney-shaped masses on crystalline calcite with galena and sphalerite. Large kidney-shaped accumulations of native arsenic with a concentric shell-like structure were found on the left bank of the river. Chikoya (Transbaikalia). In paragenesis with it, only calcite was observed in the form of rims on the walls of thin veins cutting across ancient crystalline schists. In the form of fragments (Fig. 76), arsenic was also found in the area of st. Jalinda, Amurskaya railway etc. and in other places.
In a number of deposits in Saxony (Freiberg, Schneeberg, Annaberg, etc.), native arsenic was observed in association with arsenic compounds of cobalt, nickel, silver, native bismuth, etc. All these and other finds of this mineral are of no practical significance.
Diagnosis of poisoning
Basically, the presence of arsenic in the body is diagnosed using laboratory tests. When it is present in the blood, hemoglobin decreases and the number of leukocytes decreases or increases. Urinalysis reveals hematuria, proteinuria, and the presence of casts. In chronic stages, sites of arsenic accumulation can be identified using x-ray examination.
Possible consequences
Since arsenic affects all organs and systems, most often after poisoning the functioning of the liver, kidneys and lungs is disrupted. In severe cases, due to the effect on the nervous system, the patient may remain disabled. Severe poisoning often results in death.